Bupropion-dextromethorphan (BUP-DXM) has been recently approved for the treatment of major depressive disorder.1 Clinicians should be aware of a potential interaction between DXM and selective serotonin reuptake inhibitors (SSRIs)—especially those that inhibit cytochrome P450 (CYP) 2D6. Toxicity may manifest in serotonin syndrome and DXM toxicity.
Case Report
A 68-year-old Caucasian male with a history of major depressive disorder, benign prostatic hyperplasia, and chronic multisite pain taking fluoxetine 80 mg and prazosin 5 mg nightly started BUP 105 mg/DXM 45 mg daily for continued depressive symptoms in August 2023.
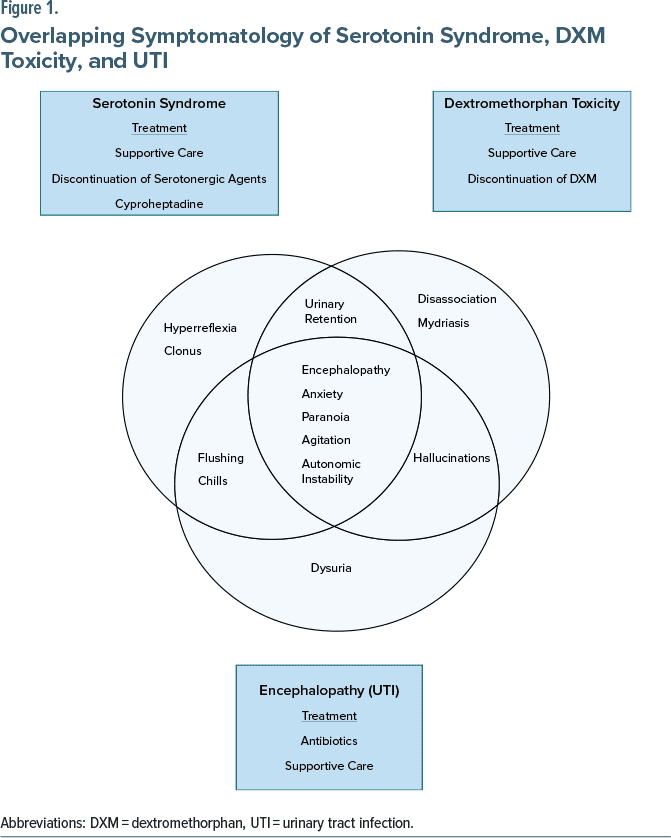
After 3 days, the patient experienced progressive flushing, chills, confusion, urinary retention, dysuria, agitation, paranoia, anxiety, and auditory/visual hallucinations of humming and persons in his home. He noted dissociation, recalling “concentrating on who I was.” He had no history of urinary tract infection (UTI), delirium, or psychosis. Given his mental status, he was unable to give a precise timeline of symptoms he experienced first.
Despite discontinuation of BUP/DXM, symptoms continued for 5 days, prompting presentation to the emergency department. He denied suicidality or having overdosed on medications. Mental status examination evinced difficulty with concentration tasks; he was oriented to self, date, situation, and location.
Upon presentation, temperature, pulse, and respirations were within normal limits. Systolic blood pressure was elevated into the 150s (the patient’s baseline). Physical examination was significant for reactive pupils with mydriasis, nystagmus, and hyperreflexia 3+/multiple beats of inducible clonus in the lower extremities. Urinalysis showed positive leukocyte esterase and >100 white blood cells. Complete blood cell count and comprehensive metabolic profile (CMP) were unremarkable. Lipase was elevated at 63. Electrocardiogram showed QTc within normal limits. Lab evaluation for levels of DXM, BUP, and pharmacogenomics was sent out. The DXM level was 10 ng/mL. BUP was undetectable. The pharmacogenomic report showed normal CYP2D6 activity.
Hospital course. Psychiatry service discontinued fluoxetine. Toxicology diagnosed a mixed picture of DXM toxicity and serotonin syndrome. The UTI complicated the diagnostic picture, as these conditions have overlapping manifestations (Figure 1).2,3 The patient received intravenous (IV) antibiotics and supportive care. He remained hemodynamically stable. His mental status improved over 4 days. Two days after discharge, he still had 3+ reflexes, but no clonus. He continued to experience dizziness and flushing but felt improved.
Discussion
Literature describes serotonin syndrome in the context of supratherapeutic DXM doses/abuse,4 and there is 1 report of appropriately consumed promethazine-DXM and escitalopram leading to presumed serotonin syndrome.5 There were no results for a literature search conducted in September 2023 with keywords “Dextromethorphan,” “Bupropion,” “SSRI,” and “Serotonin Syndrome” describing a presentation as described above.
DXM is primarily metabolized by CYP2D6 with a half-life of 2–4 hours. Both BUP and fluoxetine inhibit this enzyme.4 Approximately 10% of Caucasian patients are poor metabolizers of CYP2D6.6
Based on the pharmacokinetics of DXM, without inhibition, it would not be expected to be detectable in plasma after 5 days.7 Notably, the lab did not test for DXM metabolites.*
Mechanistically, DXM decreases glutamate transmission through inhibitory effects on N-methyl-D-aspartate (NMDA) receptors.† DXM and fluoxetine compete for binding to serotonin transporters (SERT) and σ1 receptors.‡ DXM also binds to the PCP site, an NMDA channel named given the high affinity of phencyclidine, consistent with effects as a dissociative and hallucinogen at higher doses.8,9
To this author’s knowledge, this is the first case report of combined DXM toxicity and serotonin syndrome from therapeutic doses of SSRI, BUP, and DXM. The patient’s prolonged symptomatology was likely due to fluoxetine’s long half-life, and it is possible that the clinical course may have been shorter with a different serotonergic agent/CYP2D6 inhibitor. Clinicians should be aware of this potential drug-drug interaction and consider CYP2D6 genotyping or a fluoxetine “washout.”
*DXM and its metabolites undergo renal elimination.7
†Additionally, it works as a nonselective serotonin-norepinephrine reuptake inhibitor and as a σ1 agonist.8
‡Werling et al compared the binding profiles of DXM and fluoxetine and found that they both compete for binding to serotonin transporters (SERT) and σ1 receptors with moderate to high affinity. At SERT, DXM has Ki = 40 nM compared to fluoxetine with Ki = 0.81 nM. Additionally, DXM and fluoxetine each have affinity for the 5-HT1B/D receptor. At σ1 receptors, DXM has Ki = 150 nM while fluoxetine has Ki = 214.9
Article Information
Published Online: April 3, 2024. https://doi.org/10.4088/JCP.23cr15139
© 2024 Physicians Postgraduate Press, Inc.
J Clin Psychiatry 2024;85(2):23cr15139
Submitted: October 5, 2023; accepted February 5, 2024.
To Cite: Singh MA, Johnson D. Serotonin syndrome and dextromethorphan toxicity caused by drug-drug interaction between fluoxetine and bupropion-dextromethorphan: a case report. J Clin Psychiatry. 2024;85(2):23cr15139.
Author Affiliations: VA San Diego, San Diego, California (Singh); Department of Psychiatry, University of California, San Diego, California (Johnson).
Corresponding Author: Michelle Anjali Singh, DO, VA San Diego, 3350 La Jolla Village Dr, San Diego, CA 92161 ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
Patient Consent: Consent was received from the parents of the patient to publish the case report, and information has been de-identified to protect anonymity.
Acknowledgments: The authors would like to thank Al Alam, MD, at VA San Diego, San Diego, California and Michael Duerden, MD, at Southern Arizona VA Healthcare System for providing feedback on this manuscript. Drs Alam and Duerden report no potential conflict of interest.
References (9)

- Iosifescu DV, Jones A, O’Gorman C, et al. Efficacy and safety of AXS-05 (dextromethorphan-bupropion) in patients with major depressive disorder: a phase 3 randomized clinical trial (GEMINI). J Clin Psychiatry. 2022;83(4):21m14345. PubMed CrossRef
- Werneke U, Truedson-Martiniussen P, Wikström H, et al. Serotonin syndrome: a clinical review of current controversies. J Integr Neurosci. 2020;19(4):719–727. PubMed CrossRef
- Journey JD, Agrawal S, Stern E. Dextromethorphan Toxicity. In StatPearls [Internet]. StatPearls Publishing. 2023. Updated June 26, 2023. Accessed October 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK538502/ PubMed
- Schwartz AR, Pizon AF, Brooks DE. Dextromethorphan-induced serotonin syndrome. Clin Toxicol (Phila). 2008;46(8):771–773. PubMed CrossRef
- Dy P, Arcega V, Ghali W, et al. Serotonin syndrome caused by drug to drug interaction between escitalopram and dextromethorphan. BMJ Case Rep. 2017;2017:bcr2017221486. PubMed CrossRef
- Zhou SF, Liu JP, Chowbay B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev. 2009;41(2):89–295. PubMed CrossRef
- Pope LE, Khalil MH, Berg JE, et al. Pharmacokinetics of dextromethorphan after single or multiple dosing in combination with quinidine in extensive and poor metabolizers. J Clin Pharmacol. 2004;44(10):1132–1142. PubMed CrossRef
- Nguyen L, Robson MJ, Healy JR, et al. Involvement of sigma-1 receptors in the antidepressant-like effects of dextromethorphan. PLoS One. 2014;9(2):e89985. PubMed CrossRef
- Werling LL, Keller A, Frank JG, et al. A comparison of the binding profiles of dextromethorphan, memantine, fluoxetine and amitriptyline: treatment of involuntary emotional expression disorder. Exp Neurol. 2007;207(2):248–257. PubMed CrossRef
This PDF is free for all visitors!