Bipolar affective disorder (BPAD) is a chronic episodic psychiatric illness with a lifetime prevalence of 1%–5%.1 Treatment includes antipsychotics and mood stabilizers, among which lithium has shown remarkable therapeutic benefits.2 First reported by John Cade in 1949 and approved by the US Food and Drug Administration in 1970 for acute mania and in 1974 for maintenance therapy,3 lithium offers significant benefits despite a narrow therapeutic index. Cutaneous adverse effects of lithium are less studied, with reported prevalence of 3.4%–45%.4–6 Cutaneous reactions include acneiform eruption, exfoliative dermatitis, pityriasis versicolor, dermatitis herpetiformis, hyperpigmentation, follicular keratitis, rash, urticaria, alopecia, and hidradenitis suppurativa.7 Acute rash is rare, with only a few case reports.8–11 Here, we report the case of a BPAD patient who developed a lithium-induced rash that did not recur on rechallenge.
Case Report
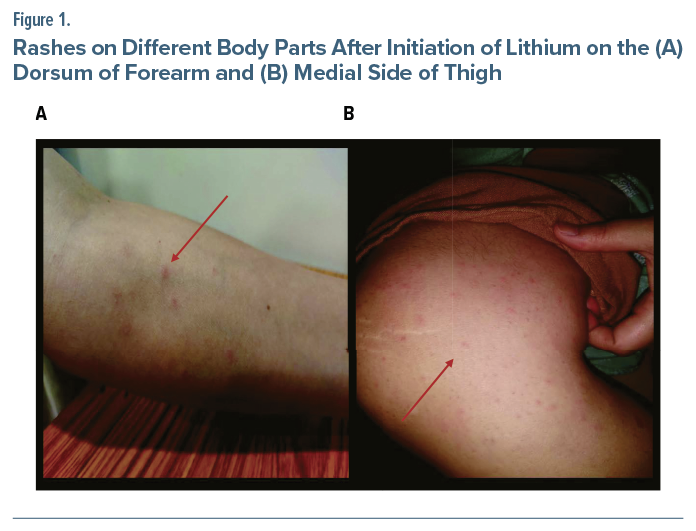
A 32-year-old woman, with no past psychiatric history or drug allergies, presented with 2 weeks of low mood, decreased interaction, decreased interest, and fatigue, followed by 1 week of elevated mood, overtalkativeness, and increased activity. Olanzapine 10 mg daily was initiated and increased to 20 mg, but mood fluctuations persisted. A diagnosis of BPAD, current episode mixed, was established. Lithium carbonate 300 mg was started. Within 12 hours, she developed maculopapular rash on her neck, chest, and axilla, later spreading to the flexor aspects of her arms, forearms, and medial sides of her thighs (Figure 1). Initially nonpruritic, the rash later became pruritic, without fever, myalgia, breathlessness, or mucosal involvement.
She continued lithium for 48 hours before presentation, after which it was discontinued. The complete blood count, absolute neutrophil count, and systemic examination were within normal limits. The dermatology team recommended continuing olanzapine; no additional treatment was given. The rash resolved spontaneously after 2 days.
The Naranjo Adverse Drug Reaction Probability Scale12 score was 8 (probable). Due to persistent mood fluctuations, olanzapine-related weight gain, and sedation, she was cross-tapered to aripiprazole, which worsened symptoms, necessitating a mood stabilizer. Lithium was rechallenged 2 weeks later and titrated to 750 mg (serum level = 0.9 mmol/L). No rash recurred, and she achieved remission. Over 12-month follow-up, she remained stable.
Discussion
Psychotropics can cause dermatological reactions ranging from mild urticaria to severe, life threatening conditions such as Stevens-Johnson syndrome, erythema multiforme, and drug reaction with eosinophilia and systemic symptoms. Other effects include angioedema, pigmentary disorders, alopecia, psoriasiform eruptions, acne, and seborrhoeic dermatitis.13 Lithium-induced acute rash is uncommon and may be pruritic or asymptomatic.14
Few cases are reported. Callaway et al8 first documented 5 cases: 4 patients had pruritic skin rashes (2 cases also with leg ulcers), and 1 patient had a leg ulcer alone. One patient’s rash recurred on rechallenge, while 2 patients’ did not.8 Kusumi9 reported 2 cases: 1 rash resolved despite continued lithium; the other resolved after discontinuation, with no recurrence on rechallenge. Sharma and Padala10 described a maculopapular rash resolving within 2 days of stopping lithium. Wang and Yang11 reported a nonitchy erythematous maculopapular rash resolving within 3 days after discontinuation. Our patient’s presentation and spontaneous resolution were similar, and she tolerated rechallenge without recurrence.
The mechanism of lithium-induced rash is unclear; type IV hypersensitivity is suspected. Lithium inhibits the phosphatidylinositol system and G-protein signaling, lowering inositol and cyclic adenosine monophosphate levels, which promote keratinocyte proliferation and neutrophil chemotaxis.14 Lithium inhibits glycogen synthase kinase-3, activating hypoxia-inducible factor-1 and increasing neutrophil, platelet, and CD-34+ cell production.15
Female sex and increased age are risk factors.5 Genetic variability in chemotaxis and neutrophil activity may influence susceptibility.11 Most rashes are self-limiting and respond to supportive care; lithium discontinuation may be required in some cases.14 Whether rechallenge is appropriate remains debated.
This case highlights that lithium-induced rash, though rare, can occur soon after initiation and resolve rapidly upon discontinuation. In selected patients, rechallenge may be feasible without recurrence, allowing continued use of lithium’s well-established mood-stabilizing benefits under close monitoring.
Article Information
Published Online: January 20, 2026. https://doi.org/10.4088/PCC.25cr04062
© 2026 Physicians Postgraduate Press, Inc.
Prim Care Companion CNS Disord 2026;28(1):25cr04062
Submitted: August 18, 2025; accepted October 9, 2025.
To Cite: Saha A, Nirisha PL, Das S, et al. Lithium-induced rash: unveiling the dermatological dilemma. Prim Care Companion CNS Disord. 2026;28(1):25cr04062
Author Affiliations: Department of Psychiatry, All India Institute of Medical Sciences, Raipur, Chhattisgarh, India (all authors).
Corresponding Author: P. Lakshmi Nirisha, MD, Department of Psychiatry, All India Institute of Medical Sciences, Raipur, 492099, Chhattisgarh, India ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
Patient Consent: Consent was received from the patient to publish the case report and images, and information has been de-identified to protect patient anonymity.
ORCID: Anirban Saha: https://orcid.org/0000-0001-6360-9866; P. Lakshmi Nirisha: https://orcid.org/0000-0003-0200-9298; Shrayasi Das: https://orcid.org/0000-0003-0656-0028; Aditya Somani: https://orcid.org/0000-0002-3283-0362
References (15)

- Bauer M, Pfennig A. Epidemiology of bipolar disorders. Epilepsia. 2005;46(Suppl 4):8–13. PubMed CrossRef
- Harwood AJ, Agam G. Search for a common mechanism of mood stabilizers. Biochem Pharmacol. 2003;66(2):179–189. PubMed CrossRef
- Price LH, Heninger GR. Lithium in the treatment of mood disorders. N Engl J Med. 1994;331(9):591–598. PubMed CrossRef
- Vestergaard P, Amdisen A, Schou M. Clinically significant side effects of lithium treatment. A survey of 237 patients in long-term treatment. Acta Psychiatr Scand. 1980;62(3):193–200. PubMed CrossRef
- Sarantidis D, Waters B. A review and controlled study of cutaneous conditions associated with lithium carbonate. Br J Psychiatry. 1983;143:42–50. PubMed CrossRef
- Chan HH, Wing Y, Su R, et al. A control study of the cutaneous side effects of chronic lithium therapy. J Affect Disord. 2000;57(1-3):107–113. PubMed CrossRef
- Yeung CK, Chan HH. Cutaneous adverse effects of lithium: epidemiology and management. Am J Clin Dermatol. 2004;5(1):3–8. PubMed CrossRef
- Callaway CL, Hendrie HC, Luby ED. Cutaneous conditions observed in patients during treatment with lithium. Am J Psychiatry. 1968;124(8):1124–1125. PubMed CrossRef
- Kusumi Y. A cutaneous side effect of lithium: report of two cases. Dis Nerv Syst. 1971;32(12):853–854. PubMed
- Sharma A, Padala PR. Lithium-induced rash. Prim Care Companion J Clin Psychiatry. 2006;8(6):377. PubMed CrossRef
- Wang EH, Yang AC. Reversible skin rash in a bipolar disorder patient on first use of lithium. Psychiatry Clin Neurosci. 2013;67(5):365. PubMed CrossRef
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. PubMed CrossRef
- Kimyai-Asadi A, Harris JC, Nousari HC. Critical overview: adverse cutaneous reactions to psychotropic medications. J Clin Psychiatry. 1999;60(10):714–726. quiz 726. PubMed
- Jafferany M. Lithium and skin: dermatologic manifestations of lithium therapy. Int J Dermatol. 2008;47(11):1101–1111. PubMed CrossRef
- Kast RE. How lithium treatment generates neutrophilia by enhancing phosphorylation of GSK-3, increasing HIF-1 levels and how this path is important during engraftment. Bone Marrow Transpl. 2008;41(1):23–26. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!