Lessons Learned at the Interface of Medicine and Psychiatry
The Psychiatric Consultation Service at Massachusetts General Hospital sees medical and surgical inpatients with comorbid psychiatric symptoms and conditions. During their twice-weekly rounds, Dr Stern and other members of the Consultation Service discuss the diagnosis and management of hospitalized patients with complex medical or surgical problems who also demonstrate psychiatric symptoms or conditions. These discussions have given rise to rounds reports that will prove useful for clinicians practicing at the interface of medicine and psychiatry.
Prim Care Companion CNS Disord 2025;27(3):24f03885
Author affiliations are listed at the end of this article.
Have you ever been uncertain about when and how you should treat a patient’s pain? Have you wondered whether and how pain can be assessed and differentiated from psychological distress? Have you struggled to decide on a strategy that eschews the use of opioids and instead emphasizes nonpharmacologic approaches to facilitate coping and improve function? If you have, the following case vignette and discussion should prove useful.
CASE VIGNETTE
Ms C, a 56-year-old woman with a body mass index (BMI) of 47 kg/m2, severe osteoarthritis, hypertension, type 2 diabetes mellitus, and fibromyalgia, sought help for chronic pain that began after a motor vehicle accident 10 years ago (in which her husband died) and which caused her to sustain a concussion, a whiplash neck injury with a C6 fracture, and an open fracture of the tibia. She has been receiving psychiatric care for posttraumatic stress disorder (PTSD), which developed after the accident. Her pain has adversely affected her quality of life and impaired her daily functioning, which led to disability. She spends most of her time in bed watching TV or reading, and she has limited social support. Her medications include metformin (1,500 mg at night), bupropion XL (300 mg daily), gabapentin (900 mg 3 times a day), and ibuprofen (800 mg 3 times a day). She also uses over-the-counter (OTC) acetaminophen as needed. Ms C went to the emergency department (ED) 7 times this year with complaints of severe neck pain, and she received short courses of oxycodone (5–7 days with a daily dose up to 30 mg).
DISCUSSION
What Is Pain, and How Is It Characterized?
Our bodies have a complex system for recognizing and reacting to pain; it involves the bidirectional interplay between the peripheral and central nervous system (CNS).1 Pain can be nociceptive pain (ie, due to tissue damaging events such as burns, crush injuries, penetrating wounds, infections, inflammation, or tissue infarction) or neuropathic pain (that is associated with nerve tissue pathology or aberrant signaling) and can be categorized as allodynia (pain from a stimulus that does not normally provoke pain), dysesthesia (abnormal and unpleasant sensations), and hyperalgesia (increased pain from a stimulus that would normally provoke pain).1
At a cellular level, pain pathways are activated by the release of myriad substances (eg, globulin, protein kinases, arachidonic acid, and substance P) that induce action potentials in medium diameter myelinated (A-δ) afferents (involved in acute, well-localized, fast pain) and small diameter unmyelinated “C” fibers (involved in poorly localized, slow pain).1,2 Regardless of its etiology, when pain lasts longer than 3 months, despite treatment, it is called chronic pain. One of the most common types of pain is neuropathic pain (eg, diabetic polyneuropathy, postherpetic neuralgia, and trigeminal neuralgia, manifest by burning, pins and needles, squeezing, or numbness), which stems from a lesion or disease that affects the somatosensory system and adversely affects one’s quality of life.3
How Can Pain Be Assessed and Quantified?
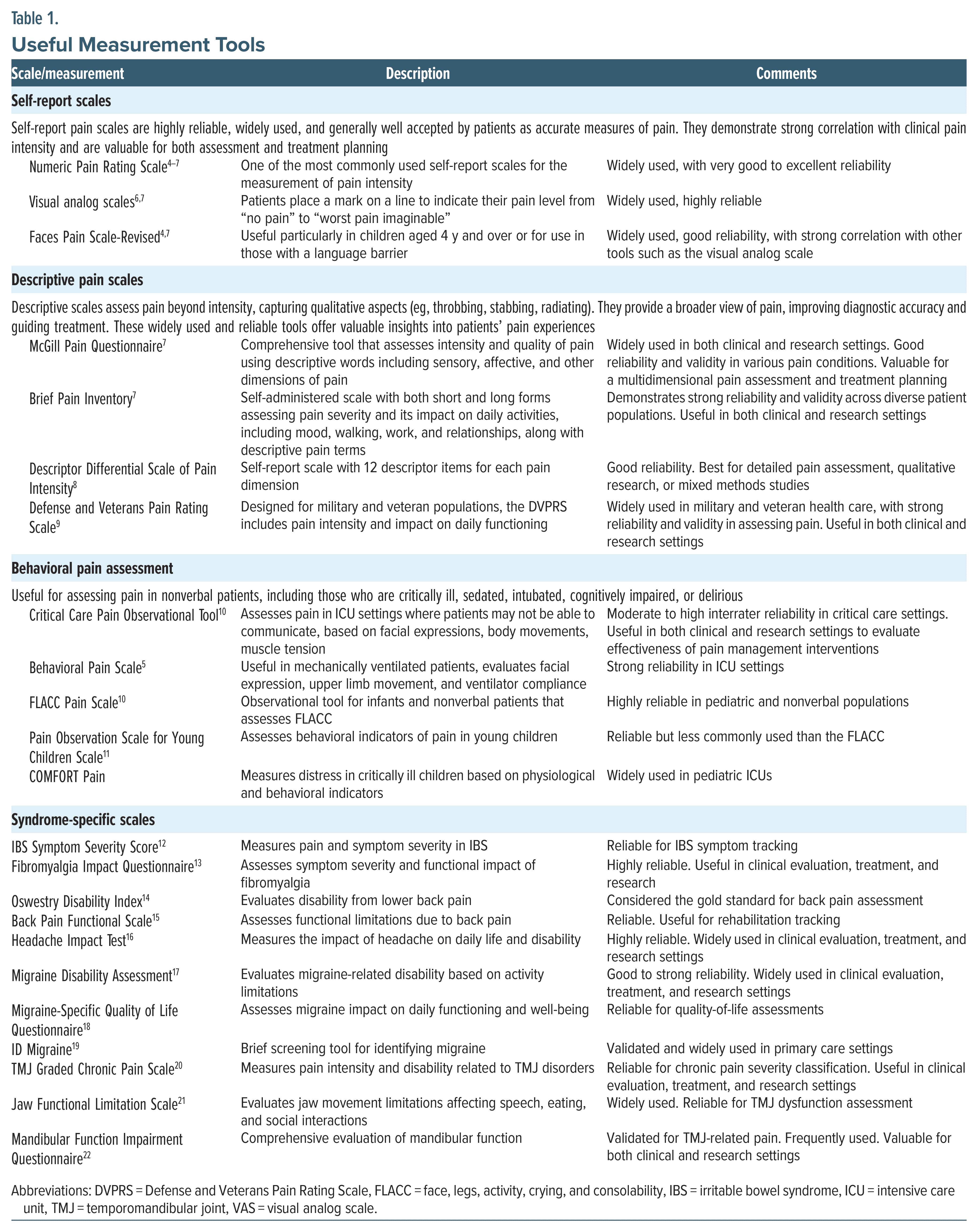
Although pain is subjective, several methods and tools are widely used to gauge pain’s intensity, quality, and impact on daily life. For a list of useful measurement tools and scales for pain disorders, including syndrome-specific options (eg, irritable bowel syndrome [IBS], fibromyalgia, migraine headaches, temporomandibular joint dysfunction, and back pain), relative reliability, and use within assessment and treatment, see Table 1.
Self-Report Scales
- Numeric rating scales: The Numeric Pain Rating Scale is one of the most commonly used self-report numeric rating scales for the measurement of pain intensity in clinical settings. This 11-point scale ranges from 0 (representing no pain) to 10 (representing the worst pain imaginable).4–7
- Visual analog scales: When using a visual analog scale, the health care provider draws a line with 2 end points, representing “no pain” to “worst pain imaginable” and asks the patient to indicate their current level of pain by placing a mark on the line.6,7
- Faces scales: The Faces Pain Scale-Revised and the Wong-Baker Pain Scale use facial expressions to indicate the level of one’s pain. They are often used with children or with those who have language barriers.4,7
Descriptive Pain Scales
- McGill Pain Questionnaire (MPQ): The MPQ has patients describe their pain from a list of 78 words (eg, throbbing, stabbing, and aching).7 This scale also allows for the rating of the pain’s intensity and quality.7 A short form of the MPQ was designed for limited use scenarios, eg, research.7
- Brief Pain Inventory (BPI): The BPI asks 15 questions that measure the intensity of pain and its interference with activities of daily living (eg, mood, walking, and sleeping).7
- Descriptor Differential Scale of Pain Intensity (DDS-I). The DDS-I assesses pain by allowing patients to rate pain intensity using descriptive terms rather than numbers.8 The DDS-I has 12 lines, each of which has a descriptor placed in the middle of the line, with a minus sign at the start of the line and a plus sign at the end of the line.
- Defense and Veterans Pain Rating Scale (DVPRS). The DVPRS enhances traditional pain assessment methods by evaluating pain intensity and its effects on daily functioning.9 This scale combines a 0–10 rating with facial expressions and color coding to convey pain levels more effectively.
Behavioral Pain Assessment
Common behavioral pain assessments include the Critical Care Pain Observation Tool; the Behavioral Pain Scale; the Face, Legs, Activity, Cry, and Consolability Pain Scale; the Pain Observation Scale for Young Children; and the COMFORT Pain Scale.5,10,11 These scales are useful for patients who cannot effectively communicate their pain (such as infants), cognitively impaired adults, or those receiving critical care. Completion of these scales involves observing behaviors (eg, facial expressions, body movements, and vocalizations).
Syndrome-Specific Scales
Numerous tools for measuring pain related to different pain disorders exist, including for IBS, fibromyalgia, back pain, migraines/headaches, and temporomandibular joint pain (see Table 1).12–22
Physiological Measures
Changes in heart rate, blood pressure, and other autonomic signs often suggest the presence of pain, although these measures are indirect and influenced by factors other than pain.
Functional Magnetic Resonance Imaging and Biomarkers
Current research is exploring the use of neuroimaging techniques and biomarkers to understand pain pathways and quantify pain objectively; these methods are primarily used in research settings and are not yet widely available in clinical practice.23
These tools, when combined with patient interviews, allow health care providers to form a more comprehensive picture of pain, which allows for a flexible approach to treatment that is based on patient needs and the clinical context.
When (and to What Extent) Should Pain Be Treated?
Pain is both a sensory and emotional experience. The concept of “pain as the fifth vital sign” was introduced to emphasize the importance of routinely assessing and managing pain. While this approach raised awareness about the need for adequate pain treatment, it also contributed to a surge in opioid prescriptions, which contributed to the opioid crisis.24 With the lessons learned from the opioid epidemic, today’s approach to pain management incorporates individualized strategies that prioritize nonopioid treatments and a holistic approach.
Most practitioners believe that pain should be treated when it adversely affects a patient’s quality of life or daily functioning. However, treatment approaches depend on the pain’s underlying cause, severity, and duration. Effective treatment of acute pain reduces the stress response, promotes healing and rehabilitation, and can prevent progression to chronic pain via central sensitization.25 Suboptimal pain control can lead to hospital readmissions and higher health care costs.26
Chronic pain (ie, pain lasting longer than 3 months) can be classified as primary (eg, as in fibromyalgia) or nonspecific (eg, as with low back pain), or secondary due to conditions or events such as cancer, neuropathy, or postsurgical trauma.27 Typically, chronic pain requires a multimodal approach to management, balancing medications with nonpharmacologic therapies (eg, physical therapy and mindfulness meditation). The extent to which pain should be treated depends on several factors, including the severity and duration of the pain, its impact on the individual’s quality of life, its underlying cause, and the patient’s overall health status.
Focusing on pain intensity alone may lead to inadequate treatment.28 Instead, treatment should prioritize improvements in the quality of life and one’s ability to engage in meaningful activities. Assessment of pain’s effect on quality of life should include psychological well-being, the quality of sleep, and the ability to perform day-to-day activities.29 As detailed previously, incorporating self-report scales to assess pain’s effect on quality of life is helpful.30 Pain management should offer adequate relief that allows the patient to achieve their individual goals and values, while considering potential adverse events.
OPIOID MEDICATIONS
What Are Opioids, How Do They Work, and How Do I Choose?
Opioids are a class of agents that includes both exogenous compounds and endogenous peptides that bind to opioid receptors in the brain and body. The endogenous opioid system modulates pain, reward, and a variety of other physiological functions, and it includes several peptide families, such as endorphins, enkephalins, dynorphins, and endomorphins. Exogenous opioids are classified as natural opiates, semisynthetic, and synthetic opioids. They can be administered orally, intravenously, intramuscularly, or transdermally. They are distributed throughout the body, with a high affinity for tissues that have rich blood supplies (eg, the brain and liver), and are primarily excreted via the kidneys.
Natural opiates are derivatives of the opium poppy (Papaver somniferum) and include morphine, thebaine, and codeine. Morphine, a highly potent opioid, is frequently used for severe pain management, while a less potent agent, codeine, is commonly used as a cough suppressant. Thebaine serves as a precursor to many semisynthetic drugs, including oxycodone, naloxone, and buprenorphine.31
Semisynthetic opioids, such as oxycodone and hydrocodone, are derived from natural opiates like thebaine and codeine. Oxycodone is prescribed for moderate-to-severe pain, while hydrocodone is used for pain relief and cough suppression. However, synthetic opioids (like fentanyl, tramadol, and methadone) are synthesized in the laboratory. Fentanyl, which is up to 100 times more potent than morphine, is commonly used in anesthesia and for severe pain management. Methadone, which is 10 times more potent than morphine, is used for pain management and for the treatment of opioid use disorder (OUD) due to its long half-life and relatively mild euphoric effects.32
The primary mechanism of action of opioids involves binding to an opioid receptor (eg, μ, δ, and κ). Opioid receptors are G-coupled receptors; activation inhibits adenylyl cyclase, which reduces the production of cyclic adenosine monophosphate (cAMP), which leads to reduced neuronal excitability. It also leads to the inhibition of presynaptic voltage-gated calcium channels, reducing calcium influx and, therefore, reducing the release of glutamate and substance P, which further diminishes pain sensation.33
Most opioids target μ receptors and produce euphoria, analgesia, and respiratory depression. The μ receptors are activated by β endorphins and endomorphins, along with full agonist opioids (FAOs).34 They are widely distributed throughout the body and play an important role in analgesia and addiction. δ receptors are activated by enkephalins, which are less associated with euphoria but may have a role in mood regulation; they play a role in modulating pain and emotional responses. The κ receptors, activated by endogenous dynorphins, induce analgesia, but their activation also induces dysphoria.35
Chronic use of opioids can lead to tolerance and withdrawal, which may result from changes to opioid receptors through desensitization, phosphorylation, internalization, and recycling. In addition, there is growing evidence for the role of neuroinflammation, glial activation, and release of proinflammatory cytokines in the development of opioid tolerance.36 For prescribed opioids, the development of tolerance and withdrawal do not count toward a diagnosis of OUD.
Selection of an opioid to mitigate pain is typically based on potency, ease of delivery, and duration of action. Side effects include reduced gastrointestinal (GI) motility, sedation, respiratory depression, tolerance, and physiological dependence (which is usually preceded by prolonged or increasingly higher doses of opioid medications).37 Among the opioids, oxycodone, hydrocodone, oxymorphone, and hydromorphone are the ones most often misused.37 Administration of opioids for the treatment of acute and severe pain should be considered after nonopioid treatment fails. They are primarily given orally, undergoing “first-pass metabolism” by the liver, which reduces serum levels, or via intravenous, transdermal, and transmucosal routes, each with a variable rate of absorption into the serum and CNS.
The Centers for Disease Control (CDC) advises prescribing opioids at the lowest effective dose for the necessary duration.38 Analgesia correlates with the opioid binding affinity. Ranging from lowest to highest is tramadol, codeine, hydrocodone, oxycodone, methadone, fentanyl, morphine, hydromorphone, oxymorphone, buprenorphine, and sufentanil. Potency is often measured in morphine milligram equivalents to guide selection.
Buprenorphine provides analgesic benefits, though it is primarily used for OUD rather than chronic pain management. Given the ceiling effect that is seen with buprenorphine on respiratory depression, it is safer than FAOs like morphine or fentanyl. While buprenorphine has a lower misuse potential than other opioids, misuse can still occur, often for self-medication or when full opioid agonists are not available in those with OUD. In addition, very low doses of buprenorphine can provide adequate to excellent analgesia.39 Buprenorphine has a high opioid receptor affinity, displacing other opioids and producing precipitated withdrawal. To avoid this, start low and increase slowly when adding buprenorphine to an FAO regimen. However, FAOs can be added to stable buprenorphine regimens for synergistic analgesia without risk of precipitated withdrawal. In addition, very low-dose naltrexone (eg, 0.5–3 mg 3 times/d) can treat pain, despite its role as an opioid antagonist by suppressing baseline receptor signaling as an inverse agonist, offering an alternative to FAO-based pain treatment.
How Quickly Does Tolerance Develop to Use of a Narcotic, and How Does Tolerance Affect Treatment?
Opioid use leads to tolerance (requiring higher doses for the same effect) and dependence (withdrawal with cessation). Tolerance and dependence to analgesic effects develop quickly, while tolerance to GI side effects is slower.40,41 The longer a patient is exposed to daily doses of an opioid, the more likely it is that tolerance and dependence will develop. To minimize this, initial prescriptions for pain relief should be limited to 4–7 days, with extended use only if benefits outweigh risks.
What Practice-Level Considerations Are Essential for Pain Management?
Clinicians must follow state laws on opioid prescribing and review Prescription Drug Monitoring Program use and regulatory requirements while implementing risk assessment protocols, toxicology screening, and treatment agreements.42 The CDC’s Clinical Practice Guidelines for Prescribing Opioids for Pain and the American Society of Addiction Medicine Consensus Statement on Drug Testing provide comprehensive guidance.42 Thorough documentation of pain assessments, treatment plans, and patient education supports patient advocacy, continuity of care, and legal protection. Toxicologic monitoring helps detect overuse or opioid substitution, prescribed or illicit. Several opioid risk tools aid in shared decision making.42,43
Can All Licensed Practitioners Prescribe Methadone or Buprenorphine for Their Outpatients?
Methadone (Schedule II) and buprenorphine (Schedule III) are indicated for OUD and pain management, but the federal regulations and prescribing rules differ. For pain management, providers must comply with the Controlled Substances Act (CSA) regulations, have an active Drug Enforcement Administration (DEA) Controlled Substance Registration Certificate, and abide by state laws.44 Methadone is prescribed for the treatment of OUDs, either medically supervised withdrawal or maintenance treatment, which is regulated under 42 CFR Part 8. Methadone for OUD is limited to facilities that are registered with the DEA as a hospital or opioid treatment program (OTP). Only providers who work at one of these accredited facilities can prescribe methadone for OUD.45 Providers within OTPs can now initiate buprenorphine and screen for initiation of methadone through telehealth. Patients no longer require 1 year of opioid addiction before being admitted to an OTP. In addition, patients are permitted to receive take-home doses within the first week of treatment, while interim treatment or “guest dosing” has been expanded from 120 to 180 days.46
Until 2023, buprenorphine for OUD required a special Drug Addiction Treatment Act (DATA 2000) waiver and training. The Consolidated Appropriations Act of 2023 removed this requirement, allowing all DEA registered providers to prescribe it for OUD without a waiver. Providers must still abide by state requirements, as well as hold an active DEA Controlled Substance Registration Certificate, as regulated under the CSA for Schedule III medications. However, as of June 27, 2023, new DEA applicants must attest to substance use disorder (SUD) educational requirements (eg, 8 hours of training, addiction medicine or addiction psychiatry board certification, or graduation within 5 years from an institution that provided a curriculum in SUD).47
How Should Acute Pain Be Managed?
Approaches to acute pain management, often centered on treatment in the ED and perioperative settings, can guide treatment across all settings.48,49 The primary objectives of managing acute pain are to provide effective pain relief, minimize medication side effects, improve patient function, and reduce the risks associated with treatment (eg, opioid misuse). Achieving these goals requires the use of a balanced approach, taking a thorough history and performing a physical examination, to assess the underlying cause and severity of the pain. This helps guide treatment decisions that are tailored to specific pain conditions (eg, due to trauma, surgery, dental work, musculoskeletal issues, muscle strain/sprain, and visceral). In addition, it is crucial to consider patient comorbidities, medication sensitivities, and the risks of misuse, especially in those with a history of alcohol or substance use disorders.
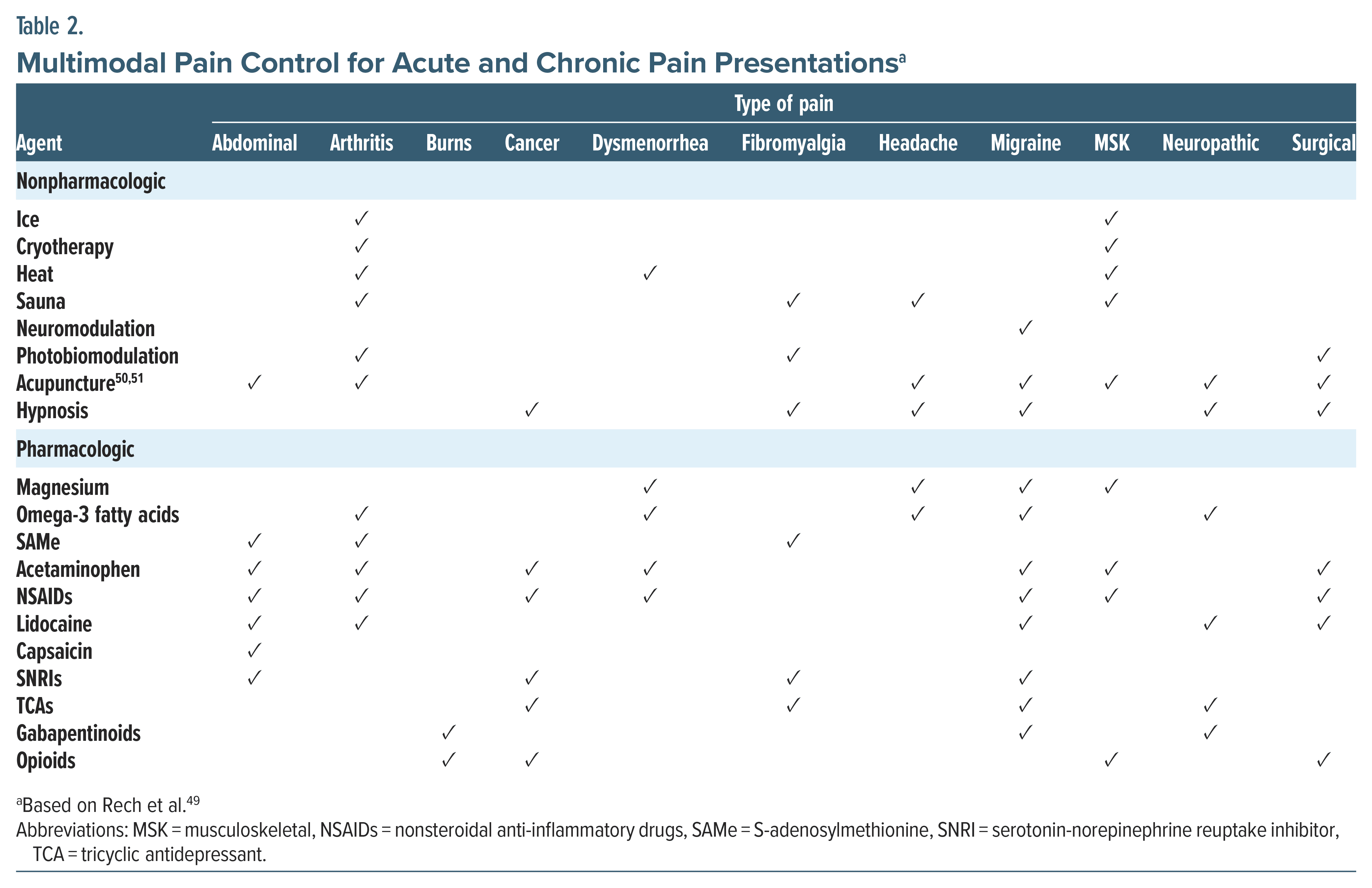
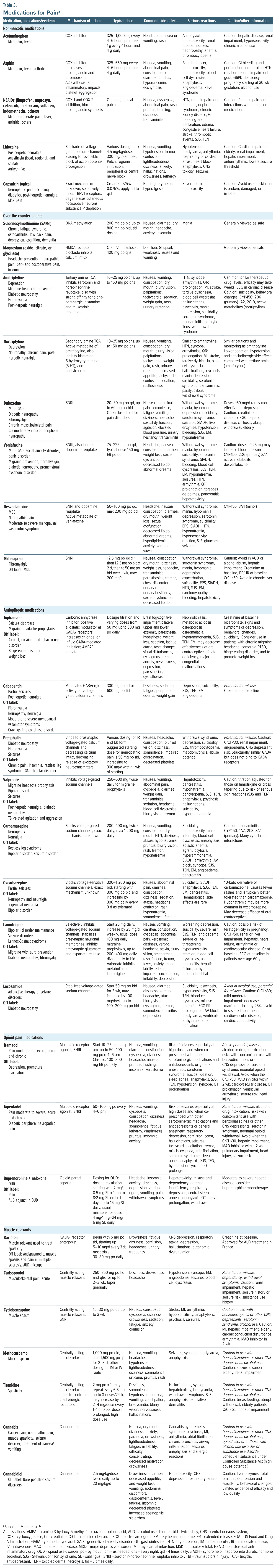
A multimodal approach that includes both nonpharmacologic and pharmacologic interventions offers significant benefits for acute pain management (Table 2). These strategies are effective for acute pain and provide relief for chronic pain. When managing acute pain, a broad range of interventions is essential to address patients’ individual needs. Both nonpharmacologic treatments (eg, neuromodulation, acupuncture, cryotherapy, and hypnosis) as well as pharmacologic options (eg, nonsteroidal anti-inflammatory drugs [NSAIDs], gabapentinoids, serotonin-norepinephrine reuptake inhibitors [SNRIs], and opioids) play vital roles in multimodal pain control (see Tables 2 and 3).37,42,48 This approach using multiple agents and therapies synergistically improves the overall efficacy of pain control while minimizing adverse side effects. Table 2 outlines a broad range of interventions for acute and chronic pain (eg, arthritis, fibromyalgia, migraine headaches, musculoskeletal, cancer, neuropathy).42 For a detailed overview of nonopioid and opioid medications, including OTC medications (eg, aspirin, acetaminophen, and NSAIDs), supplements (S-adenosylmethionine, magnesium, and capsaicin), and adjuvant medications, as well as indications and evidence, dosing, risks, and cautions, see Table 3.42
What Are Effective Approaches for Managing Chronic Pain, Including Nonpharmacologic Strategies for Pain Reduction?
Pharmacologic and nonpharmacologic pain management should be guided by a comprehensive assessment, such as the BPI as described previously.52 Integrating the World Health Organization (WHO) Analgesic Ladder with contemporary algorithms incorporates complementary and alternative medicine (CAM), OTC medications, and various interventions (see Table 2).28 The WHO Ladder includes nonopioid analgesics for mild pain, weak opioids for moderate pain, and potent opioids for severe pain, alongside adjuvants.42,53 Current approaches emphasize individualized care, multidisciplinary strategies, and the inclusion of alternative modalities like acupuncture and mindfulness-based therapies. Pharmacologic interventions remain important, with nonopioid options (eg, NSAIDs, acetaminophen, and adjuvant medications) often forming the core of treatment.42 Supplements like S-adenosylmethionine, magnesium, and capsaicin can also be useful.42 Although opioids may still have a role, particularly in cancer-related, perioperative, and dental pain, they are typically used with greater caution and monitoring in chronic pain due to the risks of long-term dependence and side effects.
NONPHARMACOLOGIC STRATEGIES
Nonpharmacologic strategies (eg, neuromodulation, physical therapy, cognitive-behavioral therapy [CBT], motivational interviewing, mindfulness, acupuncture, and other complementary therapies) have been shown to improve symptom severity in chronic pain. These treatments help reduce medication reliance, improve functional outcomes, and enhance patients’ quality of life. In addition, integrating these approaches early in treatment can help decrease pain escalation and the development of chronic pain. Moreover, these strategies empower patients by providing them with a greater sense of control over their pain, reducing distress, and improving their overall coping abilities.
Patient education and self-management are key components in the comprehensive management of chronic pain. Collaboratively engaging patients in their treatment planning, including lifestyle changes (eg, participating in regular exercise, attending to sleep hygiene, and managing stress), fosters a shared responsibility in care. This partnership enhances adherence to the treatment plan and empowers patients to manage their pain more effectively and sustainably over the long term. A coordinated effort among health care providers (eg, primary care providers, pain specialists, psychologists, psychiatrists, and physical therapists) ensures that treatment plans are individualized and based on a precision medicine approach to meet each patient’s unique needs. This integrated strategy emphasizes the continuity between acute and chronic pain management and reinforces the need for a multimodal approach across both.
ADJUVANT MEDICATIONS
Which Pharmacologic Adjuvants Can Mitigate Pain?
Adjuvant treatments for pain include various nonopioid and opioid medications that can enhance analgesic effects but also pose significant risks, including serotonin syndrome, misuse, dependence, and development of SUDs. Nonopioid medications with serotonergic properties, including selective serotonin reuptake inhibitors (SSRIs), SNRIs, tricyclic antidepressants (TCAs), ondansetron, and promethazine, may interact with opioids like buprenorphine, tramadol, and tapentadol. For dosing, side effects, indications, and interactions, see Table 3.42 Gabapentin (US Food and Drug Administration approved for postherpetic neuralgia) and other medications, such as pregabalin, lacosamide, tramadol, tapentadol, and muscle relaxants, should be used cautiously in patients taking benzodiazepines and other CNS depressants due to the risk of misuse and synergistic effects. Topiramate is effective for migraine prophylaxis and shows promise in alcohol use disorder, PTSD, and weight loss, while valproate is beneficial for migraine prevention and diabetic neuropathy, although it can elevate liver enzymes. Several other antiepileptic medications have analgesic properties. Carbamazepine, used for neuropathy and neuralgia, may induce metabolic interactions and blood dyscrasias, while oxcarbazepine is better tolerated but may cause hyponatremia. Tramadol and tapentadol can lower the seizure threshold, particularly when combined with TCAs, SSRIs, or general anesthetics. Muscle relaxants, like cyclobenzaprine, raise the risk of serotonin syndrome when prescribed with other serotonergic medications, while baclofen, a γ aminobutyric acid type B agonist, has off-label evidence for musculoskeletal pain.
Adjuvant medications play an important role in managing pain, especially in patients with comorbid mental health conditions including depression and anxiety. These medications can provide relief from pain while also targeting the psychological aspects of chronic pain, thereby improving overall patient outcomes. Muscle relaxants may alleviate muscle spasms but must be used cautiously due to risks of sedation and potential misuse. To further enhance pain management, integrating nonpharmacologic interventions, such as CBT, mindfulness, and relaxation techniques, addresses the emotional and psychological components of pain, fostering a more holistic approach to treatment.
Which Medical and Psychiatric Comorbidities Can Complicate the Assessment and Treatment of Pain?
Effective assessment and treatment of pain require a biopsychosocial approach that identifies and accounts for the impact of medical and psychiatric comorbidities. Co-occurring physical and mental health conditions contribute to more complex assessments, increased severity and perception of pain, reduced availability of treatment options, and poorer quality of life.
Obesity is strongly linked to pain, including low back pain, headaches, fibromyalgia, abdominal pain, osteoarthritis, and rheumatoid arthritis. Pain complaints increase with BMI due to increased burden on the skeletal system and joints with excess weight and mechanical stress and a proinflammatory state. In fibromyalgia, obesity heightens sensitivity to tender point palpitation.54 Obesity complicates the management of osteoarthritis, increasing surgery time for joint replacement, hospital length of stay, and analgesic use.55 Obesity prolongs recovery and increases disability in rheumatoid arthritis, limiting the patient’s engagement in physical therapy. A negative cycle of pain and inactivity worsens obesity and pain.54
Like the metabolic syndrome that occurs in obesity, individuals with diabetes are at higher risk for chronic musculoskeletal pain, neuropathy, and rheumatic problems including decreased joint mobility, tenosynovitis, carpal tunnel syndrome, and shoulder capsulitis. Diabetes can lead to degradation of connective tissue through altered blood circulation and abnormal collagen deposition in periarticular connective tissues. In addition, approximately 50% of those with diabetes develop neuropathy, and 15%–20% of these individuals experience burning or stabbing pain in their feet.56
Managing pain during pregnancy and the postpartum period is challenging, with headaches, migraines, and low back pain being most common. Pre-existing pain conditions, including rheumatoid arthritis, sickle cell disease, and Ehlers-Danlos syndrome, may worsen during pregnancy.57 Untreated chronic pain increases the risk of preterm delivery, gestational hypertension, and preeclampsia.57 Unfortunately, pain management is complicated by limited safety data on medications, as NSAIDs, venlafaxine, opioids, acetaminophen, and triptans have shown adverse effects.57 Family planning counseling should be provided to women of reproductive age receiving pain treatment.
Depression is the most well-studied psychiatric comorbidity in pain disorders, but chronic pain is more common across all psychiatric disorders, compared to those without.58 Chronic fear, avoidance, and catastrophizing worsen pain perception, lead to a fear of pain and avoidance, and worsen pain perception and treatment outcomes.58 Psychiatric illness increases substance misuse risk, limiting treatment options and causing greater impairment. Psychiatric disorders with difficulty in communication, such as autism spectrum disorder and schizophrenia, may lead to underreporting and undertreating of pain.58
Many patients with chronic pain turn to alcohol, opioids, and other substances to relieve their pain. Over time, habitual use leads to tolerance and an increased use of substances for pain relief. Although substance use may not meet criteria for an SUD initially, escalation of substance use and failure to treat pain effectively lead to a higher risk of developing an SUD. Moreover, problematic alcohol use is associated with the development and severity of several painful conditions, including pancreatitis and alcohol-related neuropathy. Studies have also shown an increased risk of developing osteoarthritis, as well as chronic pain from lower extremity injuries in those with excessive alcohol consumption.59 Management and treatment of pain is adversely impacted by substance use. In particular, the use of prescription and OTC pain medications is contraindicated with alcohol use due to the risk of GI bleeding, liver damage, and CNS depression.59 In addition, caution is required when prescribing long-term use of opioid analgesics due to the increased risk of tolerance, physical dependence, and ultimately addiction. Those with a history of OUD or another SUD may prefer to avoid opioid analgesics completely to eliminate their risk of misuse. Long-term use of opioids may also lead to hyperalgesia (ie, increased pain sensitivity). Further increasing the dose of opioids to relieve pain only results in increased pain.60
Medical and psychiatric comorbidities also require special psychopharmacologic considerations that can complicate the treatment of pain (see Table 3). Sleep disturbances including insomnia, fragmented sleep, and poor sleep quality are common in medical and psychiatric disorders, often worsening symptom severity and treatment outcomes. Chronic pain also impacts sleep, while psychiatric conditions (such as depression, anxiety, PTSD, and SUD) further contribute to sleep dysfunction in a bidirectional cycle worsening pain and emotional distress. A comprehensive evaluation including sleep assessments, incorporating CBT for insomnia, sleep hygiene interventions, and pharmacologic interventions can improve overall outcomes.
Concomitant use of multiple serotonergic agents, such as TCAs, SNRIs, tramadol, and triptans, can result in serotonin syndrome. Additionally, antidepressants that inhibit serotonin reuptake increase the risk of upper GI tract bleeding, especially when taken with NSAIDs. For those with an established cardiovascular disease, caution is required when prescribing TCAs and SNRIs. Side effects to TCAs include orthostatic hypotension, slowed cardiac conduction, arrhythmias, and an increased heart rate. SNRIs are also associated with increases in blood pressure. Many medications require dose adjustments in those with renal impairment and/or hepatic impairment. In addition, respiratory conditions can be exacerbated by opioid analgesics due to CNS depression. Several psychiatric medications are commonly used in pain treatment, and their potential to impact psychiatric comorbidities must be considered. For example, the use of antidepressants to treat pain can exacerbate comorbid bipolar disorder. Considerations for medications during pregnancy and lactation have been discussed elsewhere.61
How Can Stigma Interfere With Treatment?
Stigma, defined as “the negative social attitude attached to a characteristic of an individual that may be regarded as a mental, physical, or social deficiency,” can lead to unfair rejection, discrimination, and exclusion of an individual in several settings, including health care facilities.62 Although a variety of forms of stigma have been investigated, we will focus on 3 forms of stigma—experienced, internalized, and anticipated.63 Experienced stigma is the stigma that an individual has encountered and endured. For example, individuals with chronic pain may have been referred to as “frequent flyer, attention seekers, time wasters, drug seekers.”64 Internalized stigma occurs when an individual applies negative beliefs and stigmatized attitudes towards themselves; those with pain can internalize negative stereotypes, leading to self-judgment and blame, and questioning whether the pain is “all in their head.”64 Lastly, anticipated stigma originates from experienced and internalized stigma, which leads to the expectation of stigma and discrimination. Individuals who have internalized negative beliefs about their pain and who have experienced discrimination from health care providers are likely to expect similar stigmatizing experiences.63
Myriad mechanisms contribute to stigma surrounding pain in health care settings. Despite advances in medicine, misunderstandings remain regarding the genesis of pain. Pain’s origin is often incorrectly conceptualized as either biomedical or psychological. Further, assessment of pain is largely based on self-report and appearance of the individual. Therefore, in the absence of physical signs or a biomedical cause, pain is often viewed as psychological and associated with mental health stigma. Health care providers may also become suspicious about whether a person’s pain is genuine and question that patient’s motives.64,65 Treatment of pain may also include opioid medications, which are highly stigmatized and include stigmatizing language, such as addict, drug abuser, and junkie. These views have spread from the general public to health care settings.66
Stigmatized individuals experience worse psychological and physical well-being, as well as fewer opportunities for quality housing, employment, education, and access to health care. Experienced and anticipated stigma may lead to health care avoidance to prevent negative and stigmatizing experiences. In addition, people with internalized stigma may believe they are not worthy of being treated or that treatment will be ineffective.63,64 Unfortunately, reduced access to health care results in delayed diagnosis and treatment, a poorer prognosis, and a more difficult treatment course.
When health care is accessed, individuals who have experienced stigma secondary to pain are more likely to have their symptoms dismissed by providers or to receive inadequate treatment. Stigmatized beliefs about pain may lead providers to underassess and underestimate a patient’s complaints. Overall, women (who are more likely to be perceived as “hysterical” and suffering from a psychological problem) are more stigmatized than men, when seeking treatment for pain. As a result, women are less likely to receive analgesic prescriptions and more likely to be prescribed antidepressants. Individuals who have experienced stigma may also underreport symptoms, anticipating that they will experience discrimination or be mistreated.65
Stigma-related poor-quality health care leads to a lower quality of life and a lower satisfaction with life. Experienced, internalized, and anticipated stigma also leads to lower self-esteem and more symptoms of depression. The anticipation of stigma in social settings also leads to increased social withdrawal, avoidance, and isolation.64,67 This is especially problematic for individuals with chronic pain, who are twice as likely to make suicide attempts and to commit suicide.63 Finally, internalized stigma has been associated with catastrophic thinking regarding one’s pain and a decreased sense of control over the pain. These cognitions create a psychological barrier to recovery and result in chronicity, use of medications, longer hospital stays, and disability.65,67
Efforts should be made by health care providers to reduce their patients’ experienced, internalized, and anticipated stigma related to pain. Failing to do so interferes with access to high-quality health care, effective treatment, psychological well-being, and overall quality of life. Individuals who struggle with pain and/or substance use should be treated with respect and receive nonstigmatizing assessments and treatment.68
What Happened to Ms C?
Bupropion was tapered and discontinued, and Ms C was started on duloxetine (the dose was increased gradually to 120 mg daily), which improved her fibromyalgia symptoms. She was enrolled in a weight management program and in physical and occupational therapy. Despite losing a significant amount of weight and practicing mindfulness meditation and relaxation techniques, her neck pain persisted, which prevented her from engaging in physical exercises. She was started on buprenorphine/naloxone (2 mg 3 times a day), and the dose was increased (to 4 mg 3 times a day), which mitigated her pain and was tolerable.
CONCLUSION
Although the primary objective of managing acute pain is to provide effective pain relief, it is also important to minimize medication side effects, improve patient function, and reduce the risks associated with treatment (eg, opioid misuse). Therefore, it is crucial to consider patient comorbidities, medication sensitivities, and the risks of misuse, especially in those with a history of alcohol or SUDs. When tools (that can assess pain’s intensity, quality, and impact on daily life) are combined with patient interviews, health care providers can develop a more comprehensive picture of pain, which allows for a flexible and individualized approach to treatment that is based on their patient’s needs and the context in which they live. To guide management, the WHO Ladder includes nonopioid analgesics for mild pain, weak opioids for moderate pain, and potent opioids for severe pain, alongside adjuvants, while considering individualized care that includes alternative modalities such as acupuncture and mindfulness-based therapies.
Article Information
Published Online: June 3, 2025. https://doi.org/10.4088/PCC.24f03885
© 2025 Physicians Postgraduate Press, Inc.
Submitted: November 4, 2024; accepted February 14, 2025.
To Cite: Matta SE, Terechin O, Bonvie JL, et al. Multimodal approaches to pain management: analgesics, adjuvants, and nonpharmacologic interventions. Prim Care Companion CNS Disord 2025;27(3):24f03885.
Author Affiliations: Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts (Matta, Bonvie, Picard, Acampora, Stern); Department of Psychiatry, Brigham and Women’s Hospital, Boston, Massachusetts (Terechin); Department of Anesthesia Critical Care and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts (Acampora).
Matta, Terechin, Bonvie, Picard, and Acampora are co-first authors; Stern is senior author.
Corresponding Author: Sofia E. Matta, MD, Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, 55 Fruit St, Boston, MA 02114 ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
Clinical Points
- The concept of “pain as the fifth vital sign” was introduced to emphasize the importance of routinely assessing and managing pain. While this approach raised awareness about the need for adequate pain treatment, it also contributed to a surge in opioid prescriptions, which contributed to the opioid crisis.
- Although pain is subjective, pain’s intensity, quality, and impact on daily life can be assessed by self-reported scales, descriptive pain scales, behavioral pain assessments that involve observing behaviors, and physiological measures.
- Both nonpharmacologic treatments (eg, neuromodulation, acupuncture, cryotherapy, and hypnosis) as well as pharmacologic options (eg, nonsteroidal anti-inflammatory drugs, gabapentinoids, serotonin-norepinephrine reuptake inhibitors, and opioids) play vital roles in multimodal pain control.
- Selection of an opioid to mitigate pain is typically based on its potency, ease of delivery, and duration of action at the μ receptor.
- Semisynthetic opioids (eg, oxycodone and hydrocodone) are derived from natural opiates, while synthetic opioids (eg, fentanyl, tramadol, and methadone) are synthesized in the laboratory; however, caution is indicated when using fentanyl, as it is up to 100 times more potent than morphine.
- Stigmatized beliefs about pain may lead providers to underassess and underestimate a patient’s complaints.
References (68)

- Lee GI, Neumeister MW. Pain: pathways and physiology. Clin Plast Surg. 2020;47(2):173–180. PubMed CrossRef
- Chen JS, Kandle PF, Murray IV, et al. Physiology, pain [Updated 2023 Jul 24]. StatPearls [Internet]. StatPearls Publishing; 2024. Accessed May 22, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539789/.
- Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiol Rev. 2021;101(1):259–301. PubMed CrossRef
- Adeboye A, Hart R, Senapathi SH, et al. Assessment of functional pain score by comparing to traditional pain scores. Cureus. 2021;13(8):e16847. PubMed CrossRef
- Bouajram RH, Sebat CM, Love D, et al. Comparison of self-reported and behavioral pain assessment tools in critically ill patients. J Intensive Care Med. 2020;35(5):453–460. PubMed CrossRef
- Champion G, Goodenough B, von Baeyer C, et al. Measurement of pain by self report. In: Finley GA, McGrath PJ, eds. Measurement of Pain in Infants and Children. Progress in Pain Research and Management. Vol 10. IASP Press; 1998:123–160.
- Yazici Sayin Y, Akyolcu N. Comparison of pain scale preferences and pain intensity according to pain scales among Turkish patients: a descriptive study. Pain Manag Nurs. 2014;15(1):156–164. PubMed CrossRef
- Doctor JN, Slater MA, Atkinson HJ. The Descriptor Differential Scale of Pain Intensity: an evaluation of item and scale properties. Pain. 1995;61(2):251–260. PubMed CrossRef
- Polomano RC, Galloway KT, Kent ML, et al. Psychometric testing of the Defense and Veterans Pain Rating Scale a new pain scale for military population. Pain Med. 2016;17(8):1505–1519. PubMed CrossRef
- Stollings JL, Rumbaugh KA, Wang L, et al. Correlation of the Critical Care Pain Observation Tool and Numeric Rating Scale in intensive care unit patients. J Intensive Care Med. 2024;39(1):12–20. PubMed CrossRef
- Feng Z, Tang Q, Lin J, et al. Application of animated cartoons in reducing the pain of dressing changes in children with burn injuries. Int J Burns Trauma. 2018;8(5):106–113. PubMed
- Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11(2):395–402. PubMed CrossRef
- Bennett R. The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol. 2005;23(5 suppl 39):S154–S162. PubMed
- Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976). 2000;25(22):2940–2952. PubMed CrossRef
- Stratford PW, Binkley JM, Riddle DL. Development and initial validation of the back pain functional scale. Spine (Phila Pa 1976). 2000;25(16):2095–2102. PubMed CrossRef
- Kosinski M, Bayliss MS, Bjorner JB, et al. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res. 2003;12(8):963–974. PubMed CrossRef
- Stewart WF, Lipton RB, Dowson AJ, et al. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology. 2001;56(6 suppl 1):S20–S28. PubMed CrossRef
- Rendas-Baum R, Bloudek LM, Maglinte GA, et al. The psychometric properties of the Migraine-Specific Quality of Life Questionnaire version 2.1 (MSQ) in chronic migraine patients. Qual Life Res. 2013;22(5):1123–1133. PubMed CrossRef
- Lipton RB, Serrano D, Buse DC, et al. Improving the detection of chronic migraine: development and validation of Identify Chronic Migraine (ID-CM). Cephalalgia. 2016;36(3):203–215. PubMed CrossRef
- Sharma S, Kallen MA, Ohrbach R. Graded Chronic Pain Scale: validation of 1-month reference frame. Clin J Pain. 2021;38(2):119–131. PubMed CrossRef
- Ohrbach R, Larsson P, List T. The jaw functional limitation scale: development, reliability, and validity of 8-item and 20-item versions. J Orofac Pain. 2008;22(3):219–230. PubMed
- Kropmans TJ, Dijkstra PU, van Veen A, et al. The smallest detectable difference of mandibular function impairment in patients with a painfully restricted temporomandibular joint. J Dent Res. 1999;78(8):1445–1449. PubMed CrossRef
- Zhang Z, Gewandter JS, Geha P. Brain imaging biomarkers for chronic pain. Front Neurol. 2021;12:734821. PubMed CrossRef
- Scher C, Meador L, Van Cleave JH, et al. Moving beyond pain as the fifth vital sign and patient satisfaction scores to improve pain care in the 21st century. Pain Manag Nurs. 2018;19(2):125–129. PubMed CrossRef
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 suppl):S2–S15. PubMed CrossRef
- Sinatra R. Causes and consequences of inadequate management of acute pain. Pain Med. 2010;11(12):1859–1871. PubMed CrossRef
- Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain. 2019;160(1):19–27. PubMed CrossRef
- Sullivan MD, Ballantyne JC. Must we reduce pain intensity to treat chronic pain? Pain. 2016;157(1):65–69. PubMed CrossRef
- Turk DC, Fillingim RB, Ohrbach R, et al. Assessment of psychosocial and functional impact of chronic pain. J Pain. 2016;17(9 suppl):T21–T49. PubMed CrossRef
- Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, eds. Handbook of Pain Assessment. 3rd ed. The Guilford Press; 2011:19–44.
- Berényi S, Csutorás C, Sipos A. Recent developments in the chemistry of thebaine and its transformation products as pharmacological targets. Curr Med Chem. 2009;16(25):3215–3242. PubMed
- Dematteis M, Auriacombe M, D’Agnone O, et al. Recommendations for buprenorphine and methadone therapy in opioid use disorder: a European consensus. Expert Opin Pharmacother. 2017;18(18):1987–1999. PubMed CrossRef
- Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. 2011;115(6):1363–1381. PubMed CrossRef
- Dhaliwal A, Gupta M. Physiology, opioid receptor. [Updated 2023 Jul 24]. StatPearls [Internet]. StatPearls Publishing; 2024. Accessed May 22, 2025. https://www.ncbi.nlm.nih.gov/books/NBK546642/.
- Mysels D, Sullivan MA. The kappa-opiate receptor impacts the pathophysiology and behavior of substance use. Am J Addict. 2009;18(4):272–276. PubMed CrossRef
- Zhou J, Ma R, Jin Y, et al. Molecular mechanisms of opioid tolerance: from opioid receptors to inflammatory mediators. Exper Therapeut Med. 2021;22(3):1–8.
- Vearrier D, Grundmann O. Clinical pharmacology, toxicity, and abuse potential of opioids. J Clin Pharmacol. 2021;61(suppl 2):S70–S88. PubMed CrossRef
- Dowell D, Ragan KR, Jones CM, et al. CDC Clinical Practice Guideline for Prescribing Opioids for Pain — United States, 2022. MMWR Recomm Rep. 2022;71(3):1–95. PubMed CrossRef
- Kumar R, Viswanath O, Saadabadi A. Buprenorphine. [Updated 2023 Nov 30]. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed May 22, 2025. https://www.ncbi.nlm.nih.gov/books/NBK459126/.
- Klimas J, Gorfinkel L, Fairbairn N, et al. Strategies to identify patient risks of prescription opioid addiction when initiating opioids for pain: a systematic review. JAMA Netw Open. 2019;2(5):e193365. PubMed CrossRef
- Hayhurst CJ, Durieux ME. Differential opioid tolerance and opioid-induced hyperalgesia: a clinical reality. Anesthesiology. 2016;124(2):483–488. PubMed CrossRef
- Matta SE, Terechin O, Lento RM, et al. Strategies for the treatment of pain in the context of alcohol and substance use disorders. Prim Care Companion CNS Disord. 2024;26(5):24f03763. PubMed CrossRef
- Pain Management Opioid Taper Decision Tool, A VA Clinician’s Guide. 2016. Accessed September 29, 2024. https://nida.nih.gov/publications/drugs-brainsbehavior-science-addiction/references
- Ortiz NR, Preuss CV. Controlled substance Act [Updated 2023 Mar 24]. StatPearls [Internet]. StatPearls Publishing; 2024. Accessed May 22, 2025. https://www.ncbi.nlm.nih.gov/books/NBK574544/.
- Medications for the Treatment of Opioid Use Disorder, 42 C.F.R § 8. 2024. Accessed May 22, 2025. https://www.ecfr.gov/current/title-42/chapter-I/subchapter-A/part-8#8.1
- Substance Abuse and Mental Health Services Administration. 42 CFR Part 8 Final Rule. 2024. Accessed May 22, 2025. https://www.samhsa.gov/medicationssubstance-use-disorders/statutes-regulations-guidelines/42-cfr-part-8
- Substance Abuse and Mental Health Services Administration. Waiver Elimination (MAT Act). 2023. Accessed May 22, 2025. https://www.samhsa.gov/medicationssubstance-use-disorders/waiver-elimination-mat-act
- Mariano ER, Dickerson DM, Szokol JW, et al. A multisociety organizational consensus process to define guiding principles for acute perioperative pain management. Reg Anesth Pain Med. 2022;47(2):118–127. PubMed CrossRef
- Rech MA, Griggs C, Lovett S, et al. Acute pain management in the Emergency Department: use of multimodal and non-opioid analgesic treatment strategies. Am J Emerg Med. 2022;58:57–65. PubMed CrossRef
- World Health Organization. Acupuncture: Review and Analysis Reports on Controlled Clinical Trials. World Health Organization; 2003.
- Wang H, Yang G, Wang S, et al. The most commonly treated acupuncture indications in the United States: a cross-sectional study. Am J Chin Med. 2018;9:1–33.
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief pain inventory. Ann Acad Med Singap. 1994;23(2):129–138. PubMed
- Anekar AA, Hendrix JM, Cascella M. WHO analgesic ladder. In: StatPearls [Internet]. StatPears Publishing; 2023. Accessed September 29, 2023. https://www.ncibi.nlm.nih.gov/books/NBK554435/
- Arranz L, Rafecas M, Alegre C. Effects of obesity on function and quality of life in chronic pain conditions. Curr Rheumatol Rep. 2014;16(1):390. PubMed CrossRef
- Okifuji A, Hare BD. The association between chronic pain and obesity. J Pain Res. 2015;8:399–408. PubMed CrossRef
- Aldossari KK, Shubair MM, Al-Zahrani J, et al. Association between chronic pain and diabetes/prediabetes: a population-based cross-sectional survey in Saudi Arabia. Pain Res Manag. 2020;2020:8239474. PubMed CrossRef
- Ray-Griffith SL, Wendel MP, Stowe ZN, et al. Chronic pain during pregnancy: a review of the literature. Int J Womens Health. 2018;10(10):153–164. PubMed CrossRef
- Johnston KA, Huckins LM. Chronic pain and psychiatric conditions. Complex Psychiatry. 2023;9(1–4):24–43. CrossRef
- Zale EL, Maisto SA, Ditre JW. Interrelations between pain and alcohol: an integrative review. Clin Psychol Rev. 2015;37:57–71. PubMed CrossRef
- Volkow N, Benveniste H, McLellan T. Use and misuse of opioids in chronic pain. Annu Rev Med. 2018;69:451–465. PubMed CrossRef
- Haanpää ML, Gourlay GK, Kent JL, et al. Treatment considerations for patients with neuropathic pain and other medical comorbidities. Mayo Clin Proc. 2010;85(3 suppl):S15–S25.
- American Psychological Association. APA Dictionary of Psychology. American Psychological Association; 2018. Accessed May 22, 2025. https://dictionary.apa.org/stigma.
- Earnshaw VA, Quinn DM. The impact of stigma in healthcare on people living with chronic illnesses. J Health Psychol. 2012;17(2):157–168. PubMed CrossRef
- Bean DJ, Dryland A, Rashid U, et al. The determinants and effects of chronic pain stigma: a mixed methods study and the development of a model. J Pain. 2022;23(10):1749–1764. PubMed CrossRef
- Perugino F, De Angelis V, Pompili M, et al. Stigma and chronic pain. Pain Ther. 2022;11(4):1085–1094. PubMed CrossRef
- Harsanyi H, Cuthbert C, Schulte F. The stigma surrounding opioid use as a barrier to cancer-pain management: an overview of experiences with fear, shame, and poorly controlled pain in the context of advanced cancer. Curr Oncol. 2023;30(6):5835–5848. PubMed CrossRef
- Waugh OC, Byrne DG, Nicholas MK. Internalized stigma in people living with chronic pain. J Pain. 2014;15(5):550.e1–550.e5510.
- Sowicz TJ, Compton P, Matteliano D, et al. Pain management and substance use disorders: a position statement. J Addict Nurs. 2023;34(1):5–7. CrossRef
This PDF is free for all visitors!