Prim Care Companion CNS Disord 2022;24(2):21cr02966
To cite: Hommel EL, Nagaraja D, Chakfeh E, et al. An elusive case of cerebral amyloid angiopathy: diagnostic and treatment considerations. Prim Care Companion CNS Disord. 2022;24(2):21cr02966.
To share: https://doi.org/10.4088/PCC.21cr02966
© Copyright 2022 Physicians Postgraduate Press, Inc.
aDepartment of Medicine, Division of Geriatric Medicine and Palliative Care, University of Texas Medical Branch, Galveston, Texas
bDivision of Nephrology, Southern Colorado Nephrology Associates, Pueblo, Colorado
cDepartment of Medicine, Division of Hospital Medicine, Henry Ford Hospital, Detroit, Michigan
dDepartment of Radiology, University of Texas Medical Branch, Galveston, Texas
*Corresponding author: Erin L. Hommel, MD, Rebecca Sealy 6.302, 301 University Blvd, Galveston, TX 77555-0177 ([email protected]).
Cerebral amyloid angiopathy (CAA) results from amyloid-β protein deposition in the walls of intracerebral small arteries. It is a disease of old age, apparent on autopsy in 34% of patients over age 85 years.1 Although frequently asymptomatic, symptomatic CAA most commonly presents as spontaneous lobar hemorrhage.2 A significantly less common presentation is as a transient neurologic event (TNE).3 We present a case of TNE heralding a diagnosis of CAA in a nonagenarian.
Case Report
A 99-year-old woman with history of hypertension and mild cognitive impairment presented January 2017 to the emergency department (ED) after being found shaking with subsequent confusion at her assisted living facility. The shaking persisted for minutes according to witnesses.
In November 2015, she suffered a fall with concomitant right frontal intraparenchymal hematoma. Conservative treatment was pursued. Ten months later, she presented with a seizure-like episode similar to the current presentation. She was initiated on oxcarbazepine and had remained event free.
In the ED, she appeared confused and hyperactive. Vital signs included blood pressure 200/80 mm/Hg, which spontaneously reduced to 131/53 mm/Hg on repeat. Other vital signs were within normal limits. Besides chronic hearing loss, head and neck examinations were normal. Cardiovascular, pulmonary, and gastrointestinal examinations were benign. Neurologic examination revealed no strength or sensory deficits, but confusion complicated examination. Language testing suggested transient expressive aphasia, which resolved within minutes.
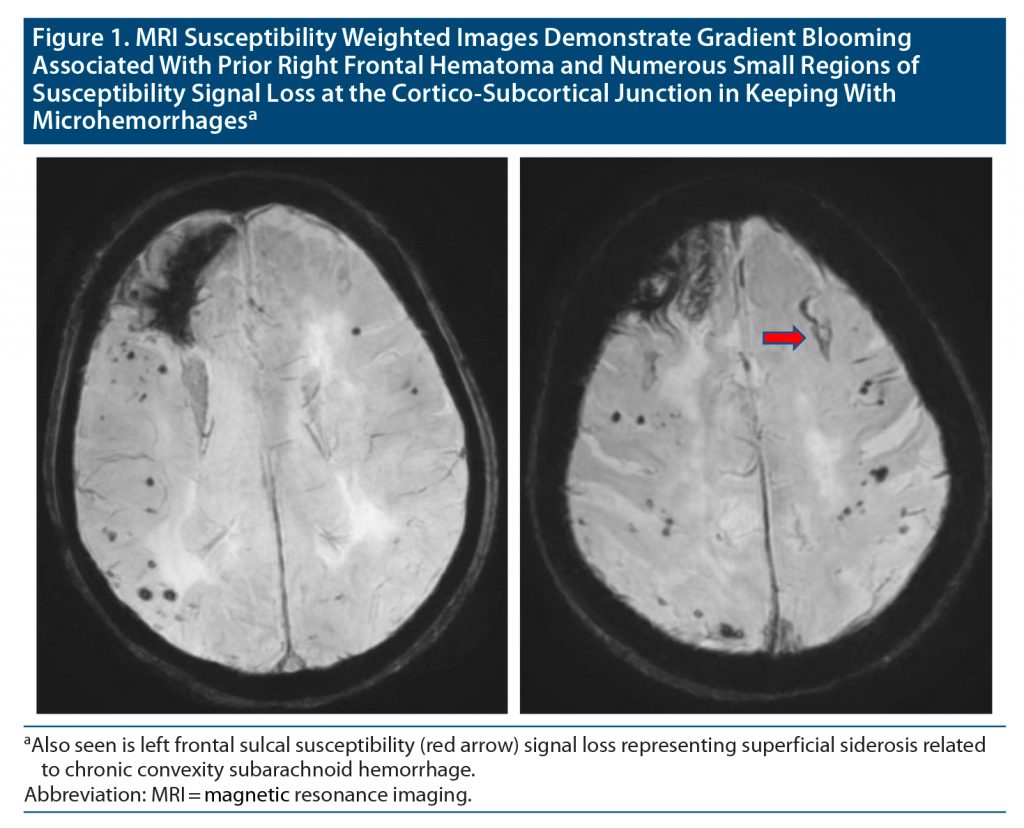
Complete blood count, comprehensive metabolic panel, and urinalysis were noncontributory as were chest x-ray and electrocardiography. Head computed tomography (CT) identified right frontal encephalomalacia consistent with prior hematoma. Electroencephalography indicated diffuse slowing, more focal in the right frontal region. She was admitted to the hospital pending brain magnetic resonance imaging (MRI) and returned to baseline after 18 hours. MRI findings confirmed her diagnosis as indicated in the Figure.
Discussion
TNE describes neurologic symptoms lasting less than 24 hours.3,4 The differential diagnosis includes transient ischemic attacks (TIA), seizure, migraine, and syncope, with TIA being most common. While the patient’s age and history of hypertension would support an ischemic cause, seizure-like episodes after a history of fall and intraparenchymal hematoma could suggest post-traumatic epilepsy.
CAA is not commonly considered in the differential diagnosis for TNE. In this case, the history of intraparenchymal hematoma could suggest prior spontaneous lobar hemorrhage. Previous brain imaging was limited to CT, which failed to provide etiology. The presence of frontal intraparenchymal hemosiderin staining along with multifocal cortical-subcortical hemosiderosis on brain MRI is highly suggestive of CAA.
CAA was traditionally diagnosed postmortem.5 Contemporary diagnostic criteria, the modified Boston criteria,6 combine imaging and clinical characteristics with pathology when available.7 Application of the criteria for probable CAA to patients with symptomatic intracerebral hemorrhage has 95% sensitivity and 81% specificity. Sophisticated MRI techniques substantially increase the diagnostic certainty.8
No disease-modifying therapies exist for CAA.8 Management hinges upon the presenting clinical syndrome. Intracerebral hemorrhage may require surgery. Rebleeding is common, suggesting a need to work up bleeding diatheses. Importantly, as in other causes of intracerebral hemorrhage, blood pressure control is critical to minimize risk of rebleeding.9 TNE requires alternative consideration.3,10 Since symptoms mimic TIA, patients often receive antithrombotic and statin therapy. Use of antithrombotics may prove catastrophic, however, as nearly 40% of patients progress to lobar hemorrhage within 2 months.3 Additionally, a link has been demonstrated between low total and low-density lipoprotein cholesterol and increased risk of intracerebral hemorrhage.11 Although this mechanism is unclear, statin therapy may be approached with caution.12 These decisions could be significantly altered by distinguishing TIA from CAA. Additionally, TNE may mimic seizures or migraines; some patients may benefit from antiepileptic or antimigraine therapy.10
CAA is an important cause of cerebral bleeding and cortical dysfunction in older adults. Recognition of its varied presentations, including as TNE, is critical for making informed diagnostic and treatment decisions.
Published online: March 22, 2022.
Potential conflicts of interest: None.
Funding/support: This work was supported by the Claude D. Pepper Older Americans Independence Center Award (no. P30-AG024832), which is funded by the National Institute on Aging (NIA), part of the United States Department of Health and Human Services.
Role of the sponsor: The sponsors had no role in the design, analysis, or interpretation of the publication. Support was restricted to publication costs only.
Previous presentation: Poster presented at the American Geriatric Society Annual Meeting; Orlando, Florida; May 3–5, 2018.
Acknowledgments: The authors thank Sarah Toombs-Smith, PhD, ELS (Sealy Center on Aging, University of Texas Medical Branch, Galveston, Texas) for writing/editing assistance. Dr Toombs-Smith has no conflicts of interest to declare.
Patient consent: Written informed consent for patient information and images was provided by the patient’s legally authorized representative, and information has been deidentified to protect patient anonymity.
References (12)

- Greenberg SM, Vonsattel JP. Diagnosis of cerebral amyloid angiopathy. sensitivity and specificity of cortical biopsy. Stroke. 1997;28(7):1418–1422. PubMed CrossRef
- Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry. 2012;83(2):124–137. PubMed CrossRef
- Charidimou A, Peeters A, Fox Z, et al. Spectrum of transient focal neurological episodes in cerebral amyloid angiopathy: multicentre magnetic resonance imaging cohort study and meta-analysis. Stroke. 2012;43(9):2324–2330. PubMed CrossRef
- Bos MJ, van Rijn MJ, Witteman JC, et al. Incidence and prognosis of transient neurological attacks. JAMA. 2007;298(24):2877–2885. PubMed CrossRef
- Greenberg SM, Charidimou A. Diagnosis of cerebral amyloid angiopathy: evolution of the boston criteria. Stroke. 2018;49(2):491–497. PubMed CrossRef
- Greenberg SM, Edgar MA. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 22-1996. Cerebral hemorrhage in a 69-year-old woman receiving warfarin. N Engl J Med. 1996;335(3):189–196. PubMed CrossRef
- Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74(17):1346–1350. PubMed CrossRef
- Yamada M. Cerebral amyloid angiopathy: emerging concepts. J Stroke. 2015;17(1):17–30. PubMed CrossRef
- Arima H, Tzourio C, Anderson C, et al; PROGRESS Collaborative Group. Effects of perindopril-based lowering of blood pressure on intracerebral hemorrhage related to amyloid angiopathy: the PROGRESS trial. Stroke. 2010;41(2):394–396. PubMed CrossRef
- Banerjee G, Carare R, Cordonnier C, et al. The increasing impact of cerebral amyloid angiopathy: essential new insights for clinical practice. J Neurol Neurosurg Psychiatry. 2017;88(11):982–994. PubMed CrossRef
- Wang X, Dong Y, Qi X, et al. Cholesterol levels and risk of hemorrhagic stroke: a systematic review and meta-analysis. Stroke. 2013;44(7):1833–1839. PubMed CrossRef
- Westover MB, Bianchi MT, Eckman MH, et al. Statin use following intracerebral hemorrhage: a decision analysis. Arch Neurol. 2011;68(5):573–579. PubMed CrossRef
This PDF is free for all visitors!