Prim Care Companion CNS Disord 2021;23(2):20l02610
To cite: Chemali Z, Biffi A, Buch K, et al. Neuropsychiatry, palliative care, and culture: what matters beyond diagnosis. Prim Care Companion CNS Disord. 2021;23(2):20l02610.
To share: https://doi.org/10.4088/PCC.20l02610
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts
bDepartment of Psychiatry, Harvard Medical School, Boston, Massachusetts
cDepartment of Radiology, Harvard Medical School, Boston, Massachusetts
dDivision of Palliative Care and Geriatric Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts
*Corresponding author: Zeina Chemali, MD, MPH, Massachusetts General Hospital, 15 Parkman St, WACC 815, Boston, MA 02114 ([email protected]).
Palliative care aims to improve the quality of life and relieve the suffering of patients with serious illness through a multidisciplinary approach attending to the physical, psychosocial, and spiritual needs of patients and their families.1 Despite this broad definition, most palliative care services have focused on patients with cancer and, in many cases, services were restricted to the end of life when curative therapies were no longer an option. In recent years with increased data on the positive impact of palliative care on quality of life and cost of care, it has been recognized as a core component of health care systems and part of comprehensive care for all patients with serious illness regardless of diagnosis, age, or prognosis.2
The integration of palliative care into neurology is not yet a standard of practice. However, this is changing. In a joint statement with the European Association for Palliative Care, the European Academy of Neurology3 called for increased collaboration between neurology and palliative care in 2016 to better address the needs of patients with neurodegenerative illness. The American Academy of Neurology also convened a committee of experts to a Neuropalliative Care Summit in 2017 to set clinical, education, and research priorities in the field.4 Neurodegenerative diseases have a wide variability in presentation, and the trajectory is usually unpredictable compared to cancer in which the disease trajectory tends to be more predictable and prognosis can be anticipated based on a patient’s functional status. Here, we review the case of a patient with an end-stage neurodegenerative diagnosis, describe the role of palliative care in our management, and discuss the cultural barriers the medical team encountered and the opportunities offered to help the patient and his family members and caregivers to define the goals of his care within their values of preserving good quality of life, respect, and dignity during the terminal phase of the patient’s illness.
Case Report
Mr A is a 60-year-old man from Qatar with a history of diabetes mellitus, hypertension, and right subdural hematoma craniotomy presenting for evaluation of progressive erratic behavior, paranoia, visual hallucinations, and memory impairment for the past year and a half. He presented as an outpatient to our hospital with his 2 sons and brother. The patient never understood why his family brought him to the hospital. He stated that he delegated all medical decision making to his older son, although he presented him as his brother. Information was collected from Mr A’s family.
The patient’s symptoms had started in Qatar 18 months before presentation to our hospital when his family first reported memory impairment and forgetting personal items. He became confused about time and place and was increasingly paranoid. He uttered nonsensical words and was restless. He saw a psychiatrist, who prescribed escitalopram, memantine, and sulpiride to help with restlessness and improve his sleep. His memory impairment continued to worsen. After 6 months, he was more paranoid and was getting lost while out by himself. He was unable to drive and could no longer execute any planning. His concentration, judgment, and problem solving were compromised. He was highly impulsive and exhibited mental rigidity. His speech alternated between mute to nonsensical to occasional periods of clarity in conveying his thoughts and wishes. With time, he could no longer tend to his camels. His mobility was preserved, and no abnormal movement was noted.
One night, the patient was found in his bed with abnormal movements and frothing at the mouth. He was taken to a nearby hospital where he was intubated. He was found to have a 13.3-mm right frontotemporal subdural hematoma (SDH) and was given mannitol. He underwent emergency craniotomy and was started on levetiracetam 500 mg twice/day. His other medications included omeprazole 40 mg daily, sulpiride 50 mg daily, and amlodipine 10 mg for 7 days; hydrochlorothiazide 25 mg; and perindopril 10 mg. He was also given vancomycin and meropenem. Following SDH evacuation, his condition worsened substantially. He was no longer attending to any executive task and often complained of headaches. Levetiracetam was discontinued after 4 weeks, and he was noted to have a Montreal Cognitive Assessment5 score of 15/30 at a geriatric psychiatry fellow outpatient visit (we do not have a copy of those results), fragmented sleep, restlessness, and isolation. He was still independent in his activities of daily living, though his family had to prompt him to eat. He was continued on memantine. After his craniotomy and during the next few months, the patient experienced visual hallucinations of cars, camels, and horses parked inside his home in Qatar. He became agitated and aggressive, requiring the family to supervise him 24/7. At that point, they decided to travel with him to the United States for consultation and treatment at our outpatient clinic. He remained under our care for the next 3 months until his departure back to Qatar.
Past medical history. Mr A’s history was notable for hypertension, diabetes mellitus, right SDH status post craniotomy and evacuation, and head trauma 12 years ago with unknown loss of consciousness. The family referred to the patient’s lifelong “hot temper” and mood changes related to the Gulf wars.
Family/social history. Mr A was married and lived mostly in the desert. He was illiterate. He was able to take care of 96 camels and attend to his family. His mother had dementia in her 90s. He has 5 brothers and 2 sisters who are healthy.
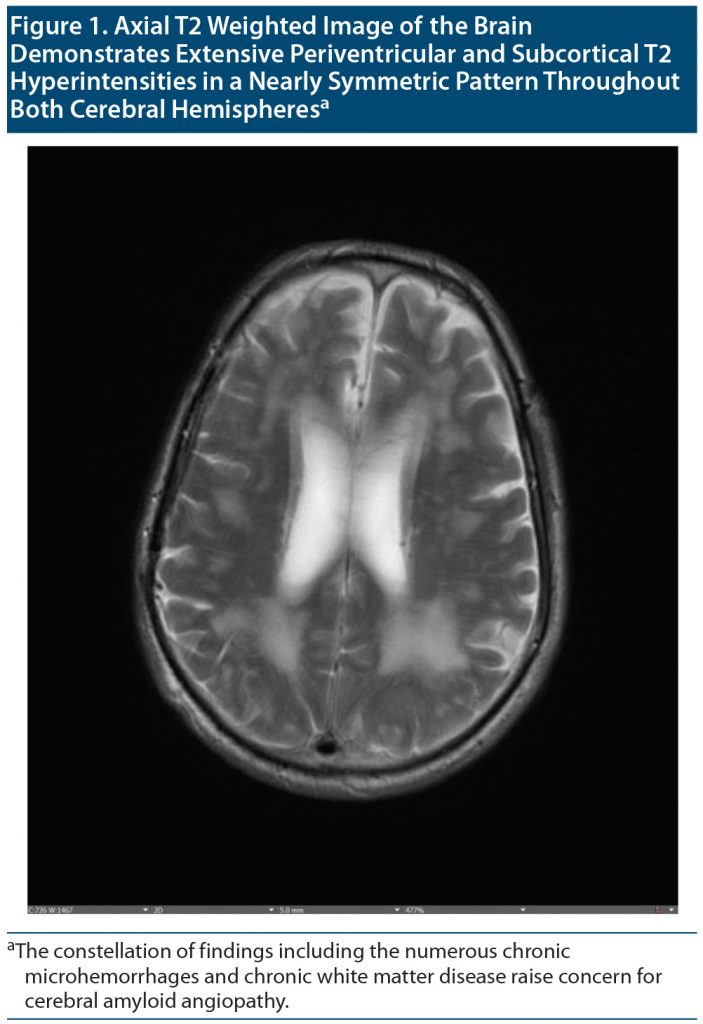
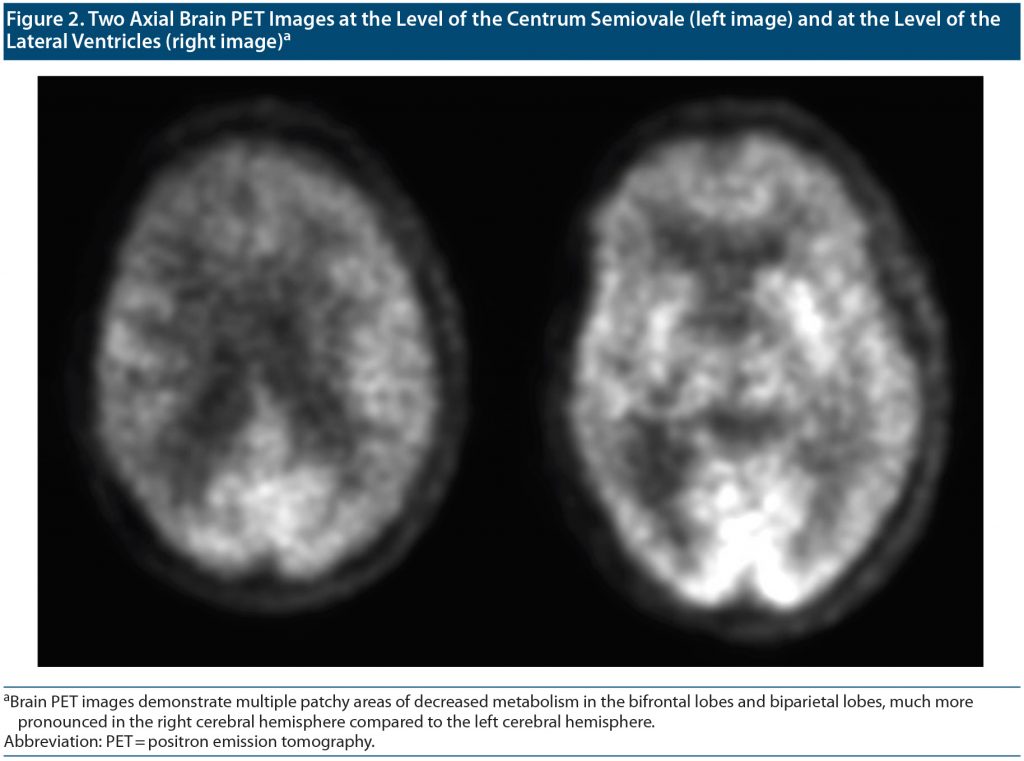
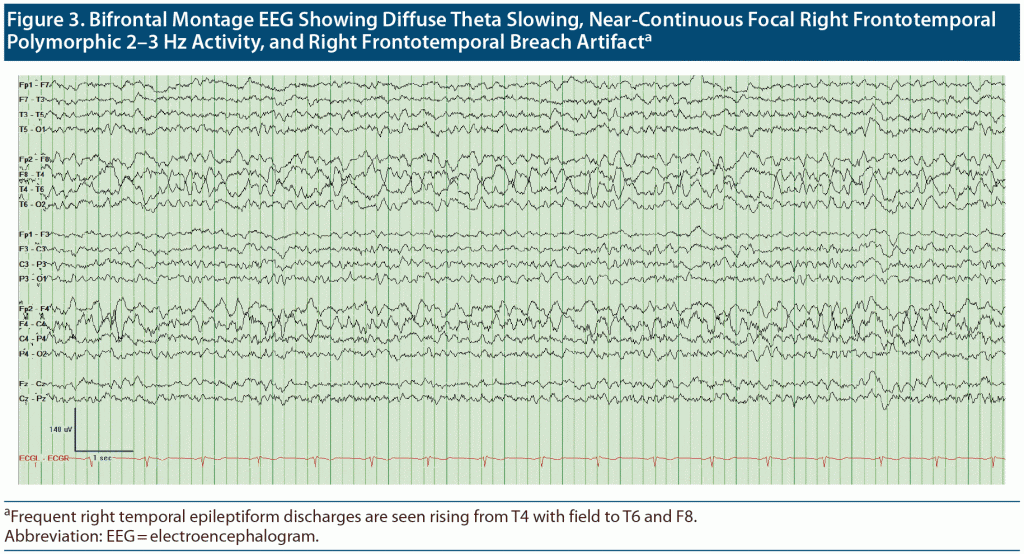
Outpatient visit course. Mr A’s cognitive status and functioning continued to deteriorate between visits to our outpatient department over a 4-month period. The patient, who at first knew his name, date of birth, and that he was from Doha, Qatar, was no longer oriented to anything. At his first visits to our clinic, it was noted that Mr A thought his kids were his brothers, though he recalled their names correctly. Given the concern for rapid deterioration, an extensive workup was initiated with repeat neuroimaging including brain magnetic resonance imaging (MRI) and functional positron emission tomography (PET) scans (Figure 1 and Figure 2, respectively) as well as electroencephalography (EEG) to rule out ongoing seizure activity. His brain MRI raised concern for cerebral amyloid angiopathy (CAA), and the brain PET scan confirmed atrophy and hypometabolism in a severe neurodegenerative pattern, ascertaining CAA as a final diagnosis. Given the frequent right temporal epileptiform discharges arising from T4 with field to T6 and F8 (Figure 3), he was placed back on levetiracetam for rapid stabilization before it was switched to valproic acid.
Additional workup included normal electrocardiogram, thyroid-stimulating hormone level, vitamin B12 level, negative rapid plasma regain, and mild anemia of unclear etiology. He was also treated for essential hypertension. ApoE status was not checked. His condition continued to worsen despite addressing the underlying seizure activity, and 4 months into treatment in our outpatient center he could no longer name any member of his family. He was perseverative and completely disoriented. He exhibited echolalia, repeating the last word of each sentence he heard. He was restless and agitated. His comprehension worsened from being able to point to the ceiling and the window to rambling speech and not being able to follow any command. He perseverated on counting backward from 10 to 1 (10, 9, 8, 9, 10, 11, 12) and could not recite days of the week backward (Saturday, Sunday, 8, 9, 10). He was unable to retain words for a memory examination. At this point, he was treated for delirium given that his seizures were under control. Levetiracetam was replaced by valproic acid sprinkles 375 mg in the morning and 500 mg at bedtime, with a therapeutic level of 72.3. Sulpiride was switched to quetiapine up to 500 mg daily in divided doses. His sleep improved, and his agitation was mostly confined to a couple of hours in the morning. However, his activities of daily living worsened, and he would not follow social norms with regard to toileting and bathing. He became incontinent of feces. He stopped praying and appeared to not know how to do it anymore. At the end of the second month of treatment, his agitation increased again alongside caregiver burnout, and melatonin 3 mg twice daily was added to help with sleep and agitation. His visits to the hospital became infrequent and short, and most of the care was done virtually with the family on the phone. They were all residing together (at least 12 family members) in a rented apartment in Boston. His oldest son was the point of contact for the care he was receiving. Although the patient’s wife traveled with him to the United States and was his primary caregiver, she was not allowed to interact with the medical team. His family took turns watching him overnight, often suffering from sleepless nights, as he was at high risk for wandering in his agitated moments. They were also overwhelmed by the need to take care of the patient in an unfamiliar environment and culture and were terrified that he might wander into a neighbor’s apartment or soil the hallway floor with his feces. Shame played a major role in the family’s dynamics, and when the team felt that the patient’s condition was terminal, communication was started about palliative options, though at first this discussion fell on deaf ears. With the patient’s worsening mental status and the heightened burnout, psychoeducation on caregiver burden was addressed first. Then, extensive discussions about palliative care offerings occurred, which were finally accepted by the family. The patient returned to Qatar with his family members, and a doctor appointed by the Qatari government accompanied them on the flight. We have no follow-up on the patient’s whereabouts or his condition after their departure. Given the severity of his disorder and the terminal stage of his illness, we believe that death would have ensued in the following 6 months.
Discussion
Cerebral amyloid angiopathy is a small vessel disease in the brain. Its hallmark is the deposition of amyloid-β (Aβ) protein within cortical and leptomeningeal blood vessel walls.6 Aβ is a normally secreted 4 kDa peptide, 40 or 42 amino acids in length, encoded by the amyloid precursor protein gene on chromosome 21. The 40-amino-acid-long Aβ (Aβ 1–40) is more soluble than the longer Aβ 1–42. Aβ 1–40 tends to deposit in artery walls in CAA, whereas Aβ1–42 is more prominent in brain plaques. CAA was recognized pathologically in the early 20th century and later was firmly associated with lobar intracerebral hemorrhage (ICH), which remains the strongest indicator of the disease.7,8 Blood-sensitive magnetic resonance sequences remain central to CAA diagnosis, visualize asymptomatic hemorrhages, and ascertain the diagnosis.9 CAA is independently associated with global rapid cognitive decline. Its associations with cognitive impairment and dementia (both with and without Alzheimer’s disease and in the context of ICH) are increasingly recognized. Presentations with predominant behavioral and psychiatric symptoms have been reported.10–12 Apo E4 status may increase the risk for developing CAA.13 Immunotherapy with ponezumab has been used in some cases despite worries of clinical deterioration.14 In our case presentation, given seizures and headache, CAA-related inflammation (CAA-ri) was considered. CAA-ri represents a rarer subset of CAA, with amyloid deposition accompanied by prominent inflammation and/or cerebral edema. Patients typically present with subacute progression of focal neurologic deficits, headaches, and seizures.15 Neuropsychiatric and neurocognitive features are usually part of the initial presentation, including memory difficulties and behavioral issues.14,15 Characteristic MRI findings include asymmetric unifocal or multifocal T2/fluid-attenuated inversion recovery hyperintense lesions in the cortex and immediate subcortical white matter, as well as subcortical hemorrhagic lesions (cerebral microbleeds, ICH, or cortical superficial siderosis). A set of validated criteria have been proposed for diagnosis of CAA-ri.16 In our case, white matter lesions were felt to be more consistent with noninflammatory CAA (given extent, symmetry, and lack of associated edema). Normal to diminished cerebral metabolism on fluorodeoxyglucose-PET also argued against an inflammatory etiology (typically presenting with focal or global hypermetabolism). As a result, a trial of immunomodulation with steroids (generally considered first-line management for CAA-ri) was not indicated. Palliative therapy with emphasis on quality of life and cultural beliefs was therefore offered.
Palliative Care in Neurologic Disorders
Neurodegenerative disorders are variable in their symptom burden and trajectory, though generally progressive and associated with significant health-related suffering in patients and caregivers. The multidisciplinary approach provided by palliative care can be invaluable in managing symptoms causing distress, providing support to caregivers, anticipating deterioration, and initiating essential communication regarding advance care planning including the assignment of health care proxies in situations wherein cognitive decline is anticipated.
The patient presented here provides an interesting illustration of this approach. Although the clinical workup eventually led to a clear diagnosis, the diagnostic clarity did not have an important impact on the clinical outcome. The course of CAA is unpredictable, and treatment has not been shown to alter the course of illness. The patient’s headaches, seizures, agitation, and insomnia were managed symptomatically. The associated cognitive and personality changes in similar patients lead to barriers in communication and high levels of existential distress in patients and their caregivers.17 Determination of goals of care and advance care planning can be particularly difficult. When patients experience fluctuations in mental status, determining mental capacity and competency and finding windows when patients are able to make decisions regarding treatment are extremely challenging. Discussions about delegation to substitute decision makers should be addressed as early as possible.
Clinical Management of Delirium
Although clinical practice guidelines on the management of delirium exist, these are predominantly based on expert opinion.18,19 The evidence on medical management remains limited. There are few clinical trials evaluating the impact of antipsychotic medications on delirium, and most have significant methodological limitations. Adequately powered studies20 evaluating the use of these medications have shown that supportive strategies are more effective in the management of delirium than pharmacologic management. Disturbances in circadian rhythm and melatonin deficiency have been postulated to play a role in the development of delirium,18 but there are no clinical trials assessing the use of melatonin in its management.
In this case, the patient was started on quetiapine primarily for its sedative effect, and melatonin was added to help with both sleep and agitation. The family also received intensive support from their physician, which included daily communication to advise on supportive interventions for management of his delirium.
Palliative Care Within a Cultural Lens
Disease understanding, the meaning of illness, and the need for developing prognostic awareness vary widely across cultures. When patients travel to receive medical care, the distance from home, family, and a familiar environment compounds the stressors associated with their medical condition. They are also less likely to have advance care planning conversations.21 Providers are reluctant to address potential negative outcomes with patients and families who have traveled in search of a “miracle cure.” Medical centers often access medical interpreters for communication and to minimize language barriers. However, this does not adequately address the cultural barriers that providers must be sensitive to in order to provide culturally competent care meeting patients’ needs. These conversations also require an understanding of interpersonal dynamics specific to cultural groups and dictate the interactions between clinician, patient, and family caregivers. For example, formal identification of a substitute decision maker is not common practice in the Middle East. Delegation of decision making generally follows the accepted social norms of a patriarchal society and goes to the male elder. Providers often experience resistance to disclosure and discussion of goals of care with the patient from family members who believe it is their duty to protect their loved one from the negative impact of bad news. Patients may feel pressured to delegate to the socially acceptable person, and delegation of alternatives may be challenged or considered offensive.
Although there was no legally designated health care proxy, once Mr A was cognitively impaired, medical decision making was automatically delegated to his eldest son according to the social norms in Qatar. Despite the wife’s presence by his side and her primary role as a caregiver, in the highly patriarchal countries of the Gulf, cultural norms dictate delegation of both communication and decision making to the eldest son. Although this is not in line with the usual practice in the West, the medical team should generally accept the decision maker identified by the family. In this particular case, discussions with the physician generally excluded his wife, who was his primary caregiver. This is despite that the treating physician was a woman. Given that supportive interventions are the mainstay of delirium management, access to the primary caregiver becomes of paramount importance, and communication through a proxy is not sufficient. The restricted access of the medical team to the primary caregiver made it impossible to assess her understanding of the situation, her ability to comprehend and implement suggested interventions, and her personal needs as a caregiver.
Conclusion
In conclusion, a palliative care multidisciplinary approach is invaluable in managing the course of neurodegenerative diseases, addressing distressing symptoms, providing support to caregivers, anticipating deterioration, and initiating essential communication in health care proxies ahead of cognitive decline.
Published online: February 24, 2021.
Potential conflicts of interest: None.
Funding/support: None.
Patient consent: Verbal consent was received from the patient’s family to publish the case report, and information has been de-identified to protect anonymity.
References (21)

- WHO Definition of Palliative Care. World Health Organization website. Accessed November 24, 2019. http://www.who.int/cancer/palliative/definition/en
- Sixty-Seventh World Health Assembly, Agenda item 15.5: Strengthening of palliative care as a component of integrated treatment within the continuum of care. World Health Organization website. Accessed November 24, 2019. http://apps.who.int/gb/ebwha/pdf_files/WHA67/A67_R19-en.pdf
- Oliver DJ, Borasio GD, Caraceni A, et al. A consensus review on the development of palliative care for patients with chronic and progressive neurological disease. Eur J Neurol. 2016;23(1):30–38. PubMed CrossRef NLM
- Creutzfeldt CJ, Kluger B, Kelly AG, et al. Neuropalliative care: priorities to move the field forward. Neurology. 2018;91(5):217–226. PubMed CrossRef NLM
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. PubMed CrossRef NLM
- Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al; Global Burden of Diseases, Injuries, Risk Factors Study 2010 (GBD 2010); GBD Stroke Experts Group. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1(5):e259–e281. PubMed CrossRef NLM
- van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9(2):167–176. PubMed CrossRef NLM
- Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2014;85(6):660–667. PubMed CrossRef NLM
- Jolink WM, Klijn CJ, Brouwers PJ, et al. Time trends in incidence, case fatality, and mortality of intracerebral hemorrhage. Neurology. 2015;85(15):1318–1324. PubMed CrossRef PubMed CrossRef NLM
- Biffi A, Bailey D, Anderson CD, et al. Risk factors associated with early vs delayed dementia after intracerebral hemorrhage. JAMA Neurol. 2016;73(8):969–976. PubMed CrossRef NLM
- Greenberg SM, Gurol ME, Rosand J, et al. Amyloid angiopathy-related vascular cognitive impairment. Stroke. 2004;35(suppl 1):2616–2619. PubMed CrossRef NLM
- Gleason A, Hayhow B, Emmanuel J, et al. Cerebral amyloid angiopathy presenting with neuropsychiatric symptoms. Aust N Z J Psychiatry. 2014;48(8):779–780. PubMed CrossRef NLM
- Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Ann Neurol. 2011;70(6):871–880. PubMed CrossRef NLM
- Chung KK, Anderson NE, Hutchinson D, et al. Cerebral amyloid angiopathy related inflammation: three case reports and a review. J Neurol Neurosurg Psychiatry. 2011;82(1):20–26. PubMed CrossRef NLM
- Yamada M. Cerebral amyloid angiopathy: emerging concepts. J Stroke. 2015;17(1):17–30. PubMed CrossRef NLM
- Auriel E, Charidimou A, Gurol ME, et al. Validation of clinicoradiological criteria for the diagnosis of cerebral amyloid angiopathy-related inflammation. JAMA Neurol. 2016;73(2):197–202. PubMed CrossRef NLM
- Gofton TE, Chum M, Schulz V, et al. Challenges facing palliative neurology practice: a qualitative analysis. J Neurol Sci. 2018;385:225–231. PubMed CrossRef NLM
- Lawlor PG, Bush SH. Delirium diagnosis, screening and management. Curr Opin Support Palliat Care. 2014;8(3):286–295. PubMed CrossRef NLM
- Bush SH, Marchington KL, Agar M, et al. Quality of clinical practice guidelines in delirium: a systematic appraisal. BMJ Open. 2017;7(3):e013809. PubMed CrossRef NLM
- Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients on palliative care: a randomized clinical trial. JAMA Intern Med. 2017;177(1):34–42. PubMed CrossRef NLM
- Partain DK, Sanders JJ, Leiter RE, et al. End-of-life care for seriously ill international patients at a global destination medical center. Mayo Clin Proc. 2018;93(12):1720–1727. PubMed CrossRef NLM
Enjoy this premium PDF as part of your membership benefits!