Pirfenidone is an antifibrotic approved for the treatment of idiopathic pulmonary fibrosis in the United States in 2014.1 The most common adverse effects (AEs) reported with pirfenidone are gastrointestinal issues and rash. Side effects such as fatigue, rash, and nausea reportedly lead to discontinuation in less than 10% of subjects.1 However, athazagoraphobia, a rare condition characterized by irrational fear of forgetting things, is not a reported side effect of this drug. We discuss a case of unusual association of pirfenidone and athazagoraphobia leading to its discontinuation in a patient with idiopathic pulmonary fibrosis.
Case Report
A 65-year-old retired man from an upper–middle-class socioeconomic background presented to the outpatient psychiatry department with the following primary concerns: repetitive thoughts and distress about forgetting things, restlessness, dysphoria, and disturbed sleep for 15 days. He had a history of interstitial lung disease for 1.5 years and was undergoing treatment with the pulmonology department. Three weeks ago, he had been started on pirfenidone 400 mg 3 times/d (1,200 mg/d). Within 7 days of initiation, he started to feel restlessness and fear of forgetting, leading to insomnia. Subsequently, a dose reduction was suggested (200 mg 3 times/d [600 mg/d]). The patient reported partial improvement in restlessness and dysphoria with dose reduction but minimal improvements in repetitive thoughts about forgetting things, which led to the referral to the psychiatry department.
On mental status examination, the patient appeared to have a tensed manner of relating, but motor behavior and speech were normal. His affect was dysphoric, but thought stream, form, and possession were normal. He reported ruminations and worry about forgetting things, with intact judgment and good insight. Bedside cognitive functions were normal, with a Montreal Cognitive Assessment (MOCA, Hindi version)2 score of 26/30, indicating minimal impairment.
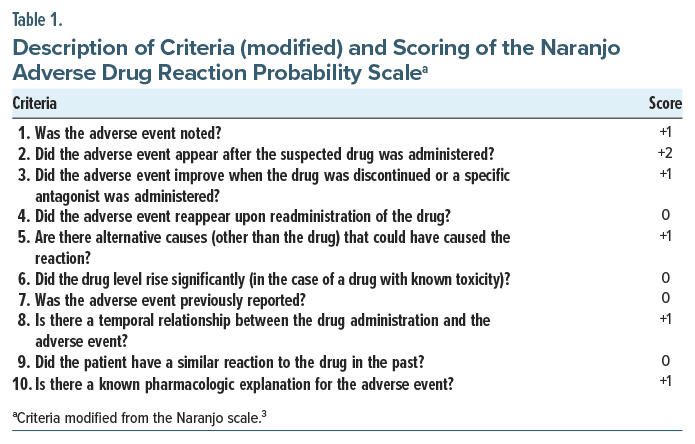
The offending drug was discontinued, citing restlessness and a short-acting pharmacologic profile, and the patient was started on tablet etizolam (0.25 mg/d) at bedtime for the first week. Given partial response, etizolam was increased to 0.75 mg/d in divided dosage, leading to improvement in restlessness, fear of forgetting, and insomnia symptoms within the next 2 weeks. The modified Naranjo Adverse Drug Reaction Probability Scale score3 was 7, indicating high probability of the symptoms due to the drug (Table 1). Finally, drug-related AEs was considered as the possible diagnosis.
Discussion
Our case indicates that pirfenidone is associated with short-term athazagoraphobia along with acute insomnia. Causality is suggested as highly probable as per the patient’s Naranjo score. A recent review of pirfenidone and related AEs suggested the median time to develop AEs ranges from 2 weeks to 3 months.1 Interestingly, in the present case, the psychological symptoms presented within 7 days. Generally, fear of forgetting4 in the elderly is considered a harbinger of ensuing neurocognitive disorders, but the cognitive screening via the MOCA was negative. The distress, fear, and ruminations led to the drug discontinuation. Drug discontinuation and intake of a short-acting anxiolytic (etizolam) were initiated together. Though etizolam might settle dysphoria and insomnia given its GABAergic properties, this would not explain the attenuation of rumination and repeated fear of forgetting. Interestingly, antifibrotic properties are due to pirfenidone modulation of cytokines and growth factors (eg, transforming growth factor β1 and b-fibroblast growth factor). Moreover, these growth factors and cytokines are involved in psychiatric disorders like depression.5
We conclude that patients taking pirfenidone should be screened for psychiatric sequelae, as this could impact compliance rates. These AE clinical profiles can aid in investigating the neuro-immunological basis of interstitial lung disease and anxiety disorders.
Article Information
Published Online: May 1, 2025. https://doi.org/10.4088/PCC.24cr03883
© 2025 Physicians Postgraduate Press, Inc.
Prim Care Companion CNS Disord 2025;27(2):24cr03883
Submitted: October 31, 2024; accepted January 8, 2025.
To Cite: Singh M, Chowdhry S, Garg S, et al. Pirfenidone induced fear of forgetting: a rare association in idiopathic pulmonary fibrosis. Prim Care Companion CNS Disord 2025;27(2):24cr03883.
Author Affiliations: Department of Psychiatry, Shri Guru Ram Rai Institute of Medical and Health Sciences, Dehradun, Uttarakhand, India (Singh, Chowdhry, Garg); Department of Psychiatry, Eras Lucknow Medical College and Hospital, Lucknow, Uttar Pradesh, India (Siddique); Department of Respiratory Medicine, Shri Guru Ram Rai Institute of Medical and Health Sciences, Dehradun, Uttarakhand, India (Rawat).
Corresponding Author: Shobit Garg, MD, DPM, Department of Psychiatry, Shri Guru Ram Rai Institute of Medical and Health Sciences, Patel Nagar, SMIH, Dehradun, Uttarakhand 248001, India ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
Patient Consent: Informed consent was obtained from the patient to publish the case report, and information has been de-identified to protect anonymity.
References (5)

- Lancaster LH, de Andrade JA, Zibrak JD, et al. Pirfenidone safety and adverse event management in idiopathic pulmonary fibrosis. Eur Respir Rev. 2017;26(146):170057. PubMed CrossRef
- Gupta M, Gupta V, Nagar Buckshee R, et al. Validity and reliability of Hindi translated version of Montreal cognitive assessment in older adults. Asian J Psychiatr. 2019;45:125–128. PubMed CrossRef
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. PubMed CrossRef
- Pinto C, Subramanyam AA. Mild cognitive impairment: the dilemma. Indian J Psychiatry. 2009;51 Suppl 1(Suppl1):S44–S51. PubMed
- Deng Z, Deng S, Zhang MR, et al. Fibroblast growth factors in depression. Front Pharmacol. 2019;10:60. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!