Trigeminal neuralgia (TN) is an exemplary condition of neuropathic facial pain.1 Approximately 15% of cases of TN are secondary, with multiple sclerosis (MS) representing 2%–11% of all cases.2 We present a case of a patient with secondary TN due to MS (TN-MS), who was minimally responsive to standard sodium channel antagonists. While responsive to opiates, the patient had a history of opioid use disorder (OUD).
Case Report
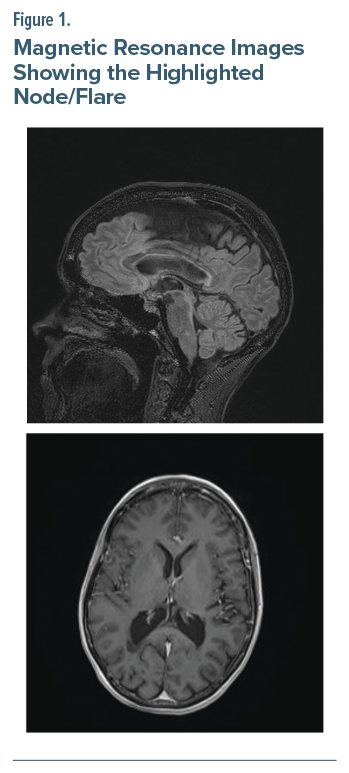
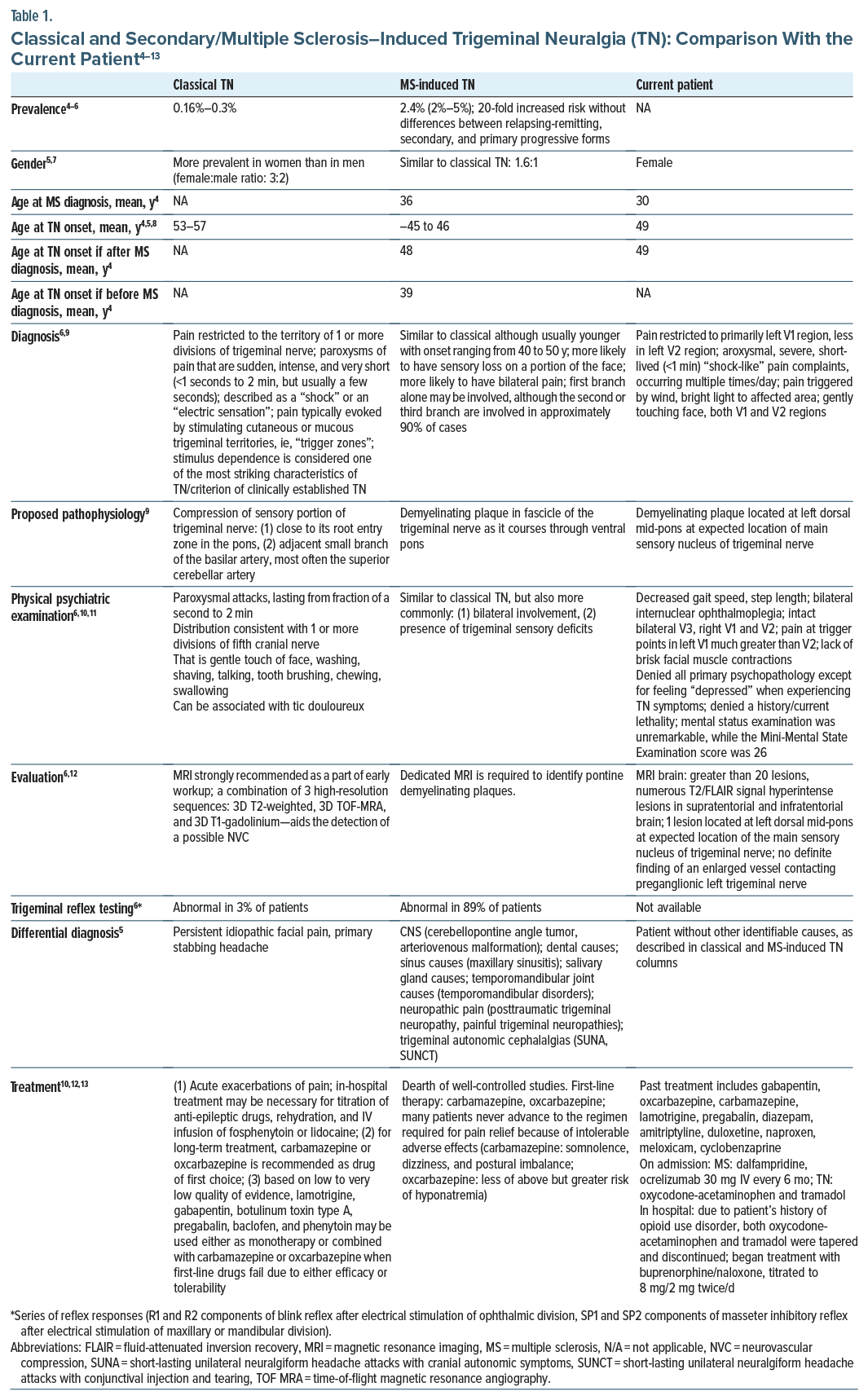
The patient was a 54-year-old woman diagnosed with MS at age 30 years. She presented to the emergency department with a 6-week history of progressively worsening left facial pain/V1 > V2 divisions. She first reported similar symptoms 5 years ago, describing the pain as severe, “electrical shock-like” lasting less than 1 minute/episode, recurring multiple times/day, with a Brief Pain Inventory-Facial3 score of 140. The patient reported short-lived attenuation of pain with carbamazepine/gabapentin and, subsequently, a variety of anticonvulsants. Magnetic resonance imaging of the brain was remarkable for numerous (>20 lesions) T2/fluid-attenuated inversion recovery signal hyperintense lesions in the supratentorial and infratentorial regions of the brain (Figure 1). One of those lesions was located at the left dorsal mid pons at the expected location of the main sensory nucleus of the trigeminal nerve. Both blood alcohol and urine drug screens were unremarkable, except for opiates/oxycodone. We were asked to evaluate the patient because of “treatment resistance.”
On psychiatric examination (Table 1),4–13 the patient admitted to a history of stimulant, opioid, alcohol, and benzodiazepine use disorder throughout her 20s, although since MS diagnosis, she only met criteria for OUD. The patient was currently prescribed oxycodone/acetaminophen but greater than 100-mg morphine equivalent. Due to the latter and OUD, the patient was transitioned and titrated to buprenorphine/naloxone 8 mg/2 mg twice daily. After 1 week of treatment, the Patient Global Impression of Change (PGIC)14 score was 3. At first-, second-, and third-month follow-up, her PGIC score was 2, and the blood alcohol and urine drug screens remained negative.
Discussion
Not atypical for MS-TN, our patient’s pain involved areas innervated by V1.6 Gently touching the face or even a slight breeze “triggered” paroxysms such that she was confined at times to wearing an eye patch over the affected eye. Similar to our patient, who was prescribed ocrelizumab, it has been reported that when demyelinating lesions develop in trigeminal sensory pathways, disease-modifying therapies have limited efficacy in TN-MS pain outcomes.15 Furthermore, there is a dearth of evidence on prevalence of μ-opioid receptor agonists (MOR-As) in TN-MS. Nonetheless, a study reported that 100% and 16% of TN-MS patients were taking opiates with and without anticonvulsants, respectively.16
In other chronic, noncancer pain syndromes, rotation to buprenorphine has not been associated with changes to patients’ pain control. In most studies, buprenorphine was associated with reduced pain severity, although the mechanism for this finding is unclear. It is possible, including in our patient, that reduced pain might be associated with buprenorphine’s unique role in opioid-induced hyperalgesia. Previous studies have reported antihyperalgesic outcomes of buprenorphine compared with other full MOR-As in animal models and humans. Participants were much more likely to complete study protocols, remain in treatment, avoid additional opioid use, and achieve analgesia if they continued to receive stable doses of buprenorphine after rotation rather than tapering off of buprenorphine. This outcome, including in our patient, was especially apparent in individuals with co-occurring OUD and chronic pain, which is consistent with the literature on OUD without chronic pain that found a greatly increased risk of return to illicit opioid use after buprenorphine taper.17
In conclusion, while uncommonly its first presentation, TN-MS is often a chronic/disabling symptom, similar to classical TN. For patients like ours with comorbid OUD and TN-MS, transition from a full MOR-A to/ continuing buprenorphine can be a successful treatment strategy, although larger studies are needed to confirm this finding.
Article Information
Published Online: October 14, 2025. https://doi.org/10.4088/PCC.25cr03985
© 2025 Physicians Postgraduate Press, Inc.
Prim Care Companion CNS Disord 2025;27(5):25cr03985
Submitted: April 16, 2025; accepted June 18, 2025.
To Cite: Spiegel DR, Milstead J, Fetter R, et al. Secondary trigeminal neuralgia due to multiple sclerosis in a patient with opioid use disorder treated with buprenorphine/ naloxone. Prim Care Companion CNS Disord 2025;27(5):25cr03985.
Author Affiliations: Department of Psychiatry and Behavioral Sciences, Macon and Joan Brock Virginia Health Sciences at Old Dominion University, Eastern Virginia Medical School, Norfolk, Virgina (all authors).
Corresponding Author: David R. Spiegel, MD, Department of Psychiatry and Behavioral Sciences, Eastern Virginia Medical School, 825 Fairfax Ave, Norfolk, VA 23507 ([email protected]).
Relevant Financial Relationships: Dr Spiegel is in the speaker’s bureau for AbbVie, Alkermes, and IntraCellular but has no conflicts of interest in the preparation of this report. The remaining authors have no disclaimer/conflicts of interest to report.
Funding/Support: None.
Patient Consent: Consent was received from the patient to publish the case report, and information has been de-identified to protect anonymity.
References (17)

- Cruccu G, Finnerup NB, Jensen TS, et al. Trigeminal neuralgia: new classification and diagnostic grading for practice and research. Neurology. 2016;87(2):220–228. PubMed CrossRef
- Latorre G, González-García N, García-Ull J, et al. Diagnosis and treatment of trigeminal neuralgia: consensus statement from the Spanish society of neurology’s headache study group. Neurol Engl Ed. 2023;38:S37–S52.
- Lee JY, Chen HI, Urban C, et al. Development of and psychometric testing for the Brief Pain Inventory-Facial in patients with facial pain syndromes. J Neurosurg. 2010;113(3):516–523. PubMed CrossRef
- Laakso SM, Hekali O, Kurdo G., et al. Trigeminal neuralgia in multiple sclerosis: prevalence and association with demyelination. Acta Neurol Scand. 2020;142(2):139–144. PubMed CrossRef
- Lambru G, Zakrzewska J, Matharu M. Trigeminal neuralgia: a practical guide. Pract Neurol. 2021;21(5):392–402. PubMed CrossRef
- Di Stefano G, Maarbjerg S, Truini A. Trigeminal neuralgia secondary to multiple sclerosis: from the clinical picture to the treatment options. J Headache Pain. 2019;20(1):20. PubMed CrossRef
- Houshi S, Tavallaei MJ, Barzegar M, et al. Prevalence of trigeminal neuralgia in multiple sclerosis:a systematic review and meta-analysis. Mult Scler Relat Disord. 2022;57:103472. PubMed CrossRef
- Fallata A, Salter A, Tyry T, et al. Trigeminal neuralgia commonly precedes the diagnosis of multiple sclerosis. Int J MS Care. 2017;19(5):240–246. PubMed CrossRef
- Crucci G, Di Stefano G, Truini A. Trigeminal neuralgia. N Engl J Med. 2020;383:754–762. PubMed
- Cruccu G, Gronseth G, Alksne J, et al. AAN-EFNS guidelines on trigeminal neuralgia management. Eur J Neurol. 2008;15(10):1013–1028. PubMed CrossRef
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. PubMed CrossRef
- Bendtsen L, Zakrzewska JM, Abbott J, et al. European Academy of Neurology guideline on trigeminal neuralgia. Eur J Neurol. 2019;26(6):831–849. PubMed CrossRef
- Rana MH, Khan AAG, Khalid I, et al. Therapeutic approach for trigeminal neuralgia: a systematic review. Biomedicines. 2023;11(10):2606. PubMed CrossRef
- Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulative Physiol Ther. 2004;27(1):26–35. PubMed CrossRef
- Mousavi SH, Lindsey JW, Gupta RK, et al. Trigeminal neuralgia in multiple sclerosis: association with demyelination and progression. Mult Scler Relat Disord. 2023;74:104727. PubMed CrossRef
- Yang AI, McShane BJ, Hitti FL, et al. Patterns of opioid use in patients with trigeminal neuralgia undergoing neurosurgery. J Neurosurg. 2019;131(6):1805–1811. PubMed CrossRef
- Powell VD, Rosenberg JM, Yaganti A, et al. Evaluation of buprenorphine rotation in patients receiving long-term opioids for chronic pain: a systematic review. JAMA Netw Open. 2021;4(9):e2124152. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!