Lumateperone is an antipsychotic medication approved by the US Food and Drug Administration in 2019 for the treatment of schizophrenia in adults.1 It exhibits a multimodal mechanism of action, functioning as a 5-hydroxytryptamine receptor 2A antagonist, a presynaptic partial agonist and postsynaptic antagonist at dopamine D2 receptors, and a serotonin reuptake inhibitor. Unlike many other antipsychotics, it has minimal affinity for histaminic H1, muscarinic M1, and adrenergic α-1 receptors, which is thought to contribute to its favorable profile regarding weight gain, metabolic disturbances, and orthostatic hypotension.1,2 This report details an instance of acute generalized urticaria in a patient immediately following the initial dose of lumateperone, a reaction that has not been extensively documented
Case Report
A 43-year-old man from a lower-middle socioeconomic stratum with a pre-existing diagnosis of hypothyroidism presented with a 12-year history of schizophrenia. His clinical picture was characterized by second-and third-person auditory hallucinations, delusions (persecutory, infidelity, and referential), fearfulness, soliloquy, unprovoked anger, and sleep disturbances. These symptoms had reportedly exacerbated over the past year. Despite an ongoing regimen of blonanserin (12 mg/d), propranolol (30 mg/d), and trihexyphenidyl (2 mg/d), persistent auditory hallucinations were the significant issue. Past treatment records reported a slurring of speech and hypersalivation with high-potency antipsychotics such as haloperidol and risperidone. No prior history of any drug-induced allergy or anaphylaxis or prior admission to a mental health facility was reported. The patient’s personal history was suggestive of continued nicotine intake in a harmful use pattern.
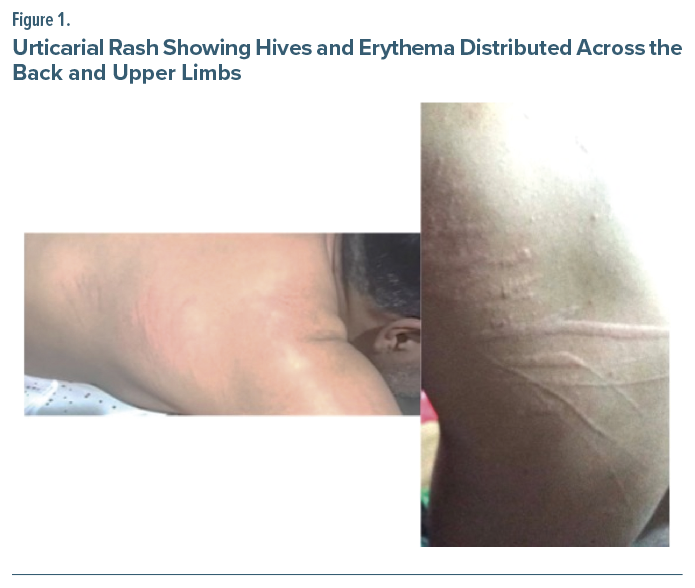
In an effort to achieve better symptom control, lumateperone 42 mg/d was introduced, while his existing medications were discontinued (12 hours before initiation). Following the first dose of lumateperone, the patient developed an acute urticarial rash (across both upper limbs and his back), specifically presenting with intense pruritus, multiple hives, generalized erythema, formation of wheals, and positive dermographism (Figure 1).
Despite this reaction, the patient took the second dose of lumateperone on the following day. This resulted in an exacerbation of the rash, with increased severity and extent of the lesions. Given the acute onset immediately following the administration of a new medication, an adverse drug reaction to lumateperone was suspected. The medication was immediately discontinued. The patient was treated with intramuscular diphenhydramine (50 mg), which led to the complete resolution of his symptoms within hours. The causality was assessed using the Naranjo Adverse Drug Reaction Probability Scale.3 The resulting score of 7 categorized this as a “probable” adverse drug reaction. The patient was subsequently started on a different antipsychotic agent with no further cutaneous reaction.
Discussion
Our patient’s score of 7 on the Naranjo Adverse Drug Reaction Probability Scale suggests a probable adverse drug reaction. The temporal relationship between the administration of lumateperone and the onset of the urticarial rash, coupled with the resolution of symptoms upon its discontinuation and treatment with an antihistamine, strongly supports this association. No other new medications, foods, or environmental exposures were identified as potential causes.4
While the incidence of urticaria in lumateperone’s preapproval clinical trials was low,5 this case highlights the potential for such cutaneous reactions in clinical practice. Lumateperone’s low affinity for histamine H1 receptors does not preclude the possibility of hypersensitivity reactions, which can be mediated by other pathways.2 Furthermore, the affinity of a drug for a receptor is distinct from its action at that receptor. An alternative consideration could be an interaction with the patient’s recently discontinued trihexyphenidyl, which has anticholinergic and some antihistaminic properties, potentially creating a complex pharmacologic state upon introduction of a newagent. Notably, lumateperone-induced urticarial rash is a listed contraindication.5
This case underscores the importance for clinicians to maintain a high index of suspicion for adverse drug reactions, even with newer medications that may have a more targeted receptor profile. Prompt recognition and withdrawal of the offending agent are critical for patient safety.
Article Information
Published Online: November 13, 2025. https://doi.org/10.4088/PCC.25cr04024
© 2025 Physicians Postgraduate Press, Inc.
Prim Care Companion CNS Disord 2025;27(6):25cr04024
Submitted: June 15, 2025; accepted August 19, 2025.
To Cite: Garg N, Garg S, Dhyani M. Probable lumateperone-associated urticarial rash in a patient with schizophrenia. Prim Care Companion CNS Disord 2025;27(6):25cr04024.
Author Affiliations: Department of Psychiatry, Shri Guru Ram Rai Institute of Medical and Health Sciences, Shri Guru Ram Rai University, Dehradun, Uttarakhand, India (all authors).
Corresponding Author: Shobit Garg, MD, DPM, Department of Psychiatry, Shri Guru Ram Rai Institute of Medical and Health Sciences, Shri Guru Ram Rai University, Dehradun, Uttarakhand, 248001 India ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
Patient Consent: Consent was received from the patient to publish the case report and photographs and information has been de-identified to protect patient anonymity.
References (5)

- Tarzian M, Ndrio M, Chique B, et al. Illuminating hope for mental health: a drug review on lumateperone. Cureus. 2023;15(9):e46143. PubMed CrossRef
- Sowa-Kućma M, Pańczyszyn-Trzewik P, Jaeschke RR. Exploring the pharmacological and clinical features of lumateperone: a promising novel antipsychotic. Int J Mol Sci. 2024;25(24):13289. PubMed CrossRef
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. PubMed CrossRef
- Deacock SJ. An approach to the patient with urticaria. Clin Exp Immunol. 2008;153(2):151–161. PubMed CrossRef
- Food and Drug Administration (FDA). CAPLYTA (lumateperone) capsules [package insert on the Internet]. Intra-Cellular Therapies, Inc.;2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/209500s009lbl.pdf.
Enjoy this premium PDF as part of your membership benefits!