Objective: Although psychiatric manifestations are one of the most common presentations of pediatric N-methyl-d-aspartate receptor (NMDAR) encephalitis, there is a lack of studies that characterize psychiatric aspects of this disorder. This study was designed to address this gap.
Methods: Initial clinical presentations including psychiatric symptoms, treatment details, and outcome with respect to psychiatric symptoms were collected from medical case records of children aged less than 18 years with seropositive NMDAR encephalitis from a single tertiary care center (May 2010-November 2016). The Brief Psychiatric Rating Scale for Children (BPRS-C) was administered at the time of presentation and at follow-up.
Results: Clinical records from 16 girls and 5 boys of whom 12 were prepubertal (< 12 years) and 9 were postpubertal (≥ 12 years) were analyzed. All 21 children presented with psychiatric symptoms at initial presentation. In 10 children (47.6%), psychiatric symptom was the first symptom. Major psychiatric symptoms included inappropriate crying (most common, 66.7%, n = 14), social withdrawal (57.1%, n = 12), unprovoked anger outburst (47.6%, n = 10), unprovoked screaming behavior (38.1%, n = 8), and talking to self irrelevantly (42.9%, n = 9). In addition to psychiatric symptoms, at least 1 of the following was also seen in all children: speech disturbance (85.7%, n = 18), seizure (85.7%, n = 18), or movement disorder (76.2%, n = 16). Mood symptoms (85.7%, n = 18) were the most common psychopathology. A comorbid psychiatric diagnosis (ICD-10 criteria) was made in 11 children (52.4%); the most common was organic mood disorder (n = 6). The mean BPRS-C score at presentation in prepubertal children was higher than that of postpubertal children (21 vs 17). After immune modulation, clinical improvement was noted after a mean ± SD of 7.4 ± 4.8 months in all 20 children followed up. Three of the 4 children with residual psychiatric symptoms and persistent academic difficulties were prepubertal.
Conclusions: Psychiatric manifestations that are usually mood related are quite common in pediatric NMDAR encephalitis. Prepubertal presentation of this disorder appears to be more severe and may lead to persistent psychiatric and cognitive symptoms.
ABSTRACT
Objective: Although psychiatric manifestations are one of the most common presentations of pediatric N-methyl-d-aspartate receptor (NMDAR) encephalitis, there is a lack of studies that characterize psychiatric aspects of this disorder. This study was designed to address this gap.
Methods: Initial clinical presentations including psychiatric symptoms, treatment details, and outcome with respect to psychiatric symptoms were collected from medical case records of children aged less than 18 years with seropositive NMDAR encephalitis from a single tertiary care center (May 2010-November 2016). The Brief Psychiatric Rating Scale for Children (BPRS-C) was administered at the time of presentation and at follow-up.
Results: Clinical records from 16 girls and 5 boys of whom 12 were prepubertal (< 12 years) and 9 were postpubertal (≥ 12 years) were analyzed. All 21 children presented with psychiatric symptoms at initial presentation. In 10 children (47.6%), psychiatric symptom was the first symptom. Major psychiatric symptoms included inappropriate crying (most common, 66.7%, n = 14), social withdrawal (57.1%, n = 12), unprovoked anger outburst (47.6%, n = 10), unprovoked screaming behavior (38.1%, n = 8), and talking to self irrelevantly (42.9%, n = 9). In addition to psychiatric symptoms, at least 1 of the following was also seen in all children: speech disturbance (85.7%, n = 18), seizure (85.7%, n = 18), or movement disorder (76.2%, n = 16). Mood symptoms (85.7%, n = 18) were the most common psychopathology. A comorbid psychiatric diagnosis (ICD-10 criteria) was made in 11 children (52.4%); the most common was organic mood disorder (n = 6). The mean BPRS-C score at presentation in prepubertal children was higher than that of postpubertal children (21 vs 17). After immune modulation, clinical improvement was noted after a mean ± SD of 7.4 ± 4.8 months in all 20 children followed up. Three of the 4 children with residual psychiatric symptoms and persistent academic difficulties were prepubertal.
Conclusions: Psychiatric manifestations that are usually mood related are quite common in pediatric NMDAR encephalitis. Prepubertal presentation of this disorder appears to be more severe and may lead to persistent psychiatric and cognitive symptoms.
Prim Care Companion CNS Disord 2017;19(4):17m02110
https://doi.org/10.4088/PCC.17m02110
© Copyright 2017 Physicians Postgraduate Press, Inc.
aDepartment of Clinical Neurosciences, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India
bDepartment of Neurology, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India
cDepartment of Neuropathology, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India
dDepartment of Child and Adolescent Psychiatry, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India
*Corresponding author: Satish Chandra Girimaji, MD, Department of Child and Adolescent Psychiatry, National Institute of Mental Health and Neurosciences, Hosur Rd, Bangalore 560 029, Karnataka, India ([email protected]).
Since the description of N-methyl-d-aspartate receptor (NMDAR) encephalitis in 2007,1 NMDAR has been increasingly identified as a frequent cause of encephalitis in the pediatric population.2 Cases3-5 of NMDAR encephalitis presenting as primary psychiatric disorder as well as mimicking neuroleptic malignant syndrome in adolescents have been reported. Although psychiatric manifestations are one of the most common presenting symptoms in pediatric NMDAR, few studies have focused on the type of psychiatric manifestations in this condition. The available studies6-10 reported mainly on frequency of psychiatric issues and did not elucidate the detailed psychiatric symptomatology, psychiatric diagnostic status, and treatment response with respect to psychiatric manifestations. Thus, this study was designed to fill this void by primarily focusing on psychiatric aspects of pediatric NMDAR encephalitis.
METHODS
This study was conducted at the National Institute of Mental Health and Neurosciences (NIMHANS), a tertiary care teaching hospital in Bangalore, India, between May 2010 and November 2016. Medical case records of confirmed cases of seropositive NMDAR encephalitis in patients aged less than 18 years (n = 21) were included in the study. Neurologic manifestations and therapeutic outcome of a part of this cohort (n = 13) were published previously.6 The following data were collected from the medical records: (1) initial clinical presentation, specifically documenting the types of psychiatric and behavioral symptoms in addition to neurologic symptoms; (2) treatment details; and (3) treatment response with respect to psychiatric manifestations. The Brief Psychiatric Rating Scale for Children (BPRS-C)11 was used at the time of presentation and at follow-up. The BPRS-C is an appropriate instrument to rate child psychopathology in medical record-based studies. The scale has 21 items, and each item is graded from zero to 6 (minimum total score: 0, maximum total score: 126). Institute ethical approval was obtained for the study. The data were analyzed using the appropriate statistical methods such as mean, median, standard deviation, range, percentage, and frequency.
RESULTS
Initial Clinical Presentation
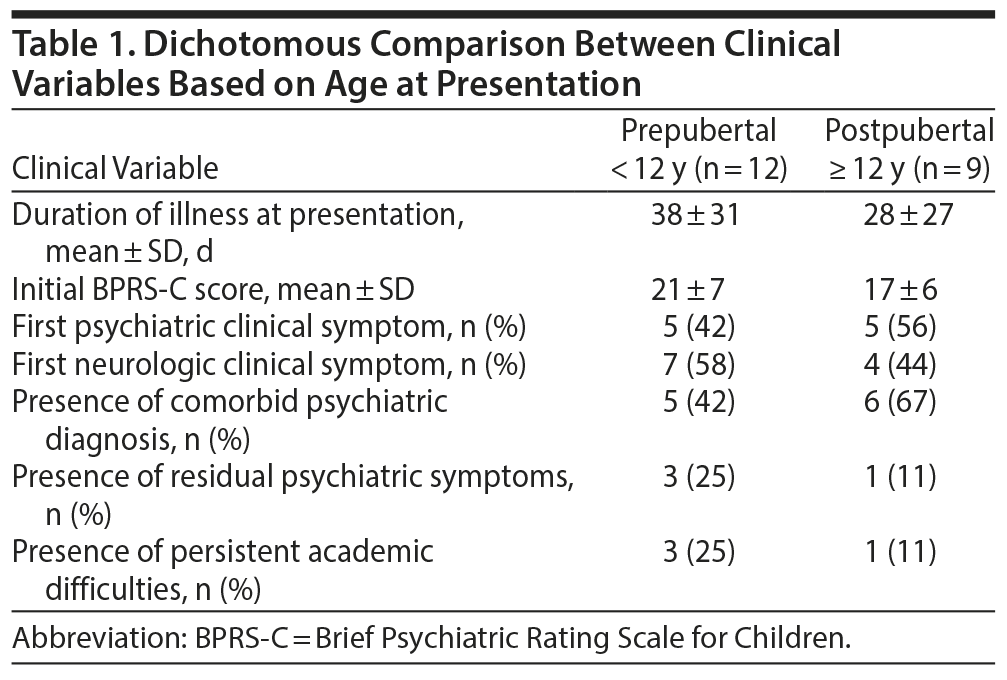
The subjects included 16 girls and 5 boys. The mean ± SD age at evaluation was 10.4 ± 4.9 years, ranging from 4 years to 18 years. There were 10 girls and 2 boys aged < 12 years (prepubertal) and 6 girls and 3 boys aged ≥ 12 years (postpubertal). Significant past history of seizure disorder was present in 3 (of 21) children, and history of similar illness in the past was present in 1 child. The median duration of illness at presentation was 30 days (range, 4-120 days). The mean duration at presentation of prepubertal children was more than that of postpubertal children (38 days vs 28 days) (Table 1). Ten children (47.6%) had fever at illness onset.
All 21 children had psychiatric symptoms at clinical presentation. In 10 children (47%), psychiatric symptom was the first symptom. The percentage of postpubertal children who first presented with a psychiatric symptom was higher than that of prepubertal children (56% vs 42%). Major psychiatric symptoms included inappropriate crying (most common, 66.7%, n = 14), social withdrawal (57.1%, n = 12), unprovoked anger outburst (47.6%, n = 10), unprovoked screaming behavior (38.1%, n = 8), talking irrelevantly to self (42.9%, n = 9), failure to recognize familiar people (38.1%, n = 8), and posturing (38.1%, n = 8). Other infrequent psychiatric symptoms included fearfulness (28.6%, n = 6), hallucinations (23.8%, n = 5), inappropriate laughing (19%, n = 4), hyperactivity (14.3%, n = 3), physical aggression (9.5%, n = 2), and compulsive behavior (4.8%, n = 1). The mean ± SD BPRS-C score at presentation was 19.3 ± 1.5 (range, 7-29). The mean BPRS-C score in prepubertal children was higher than that of postpubertal children (21 vs 17). Psychiatric symptoms were divided into 8 major domains of psychopathology. These categories included mood (85.7%, n = 18), behavioral disorganization (71.4%, n = 15), social withdrawal (57.1%, n = 12), cognitive (38.1%, n = 8), catatonic (38.1%, n = 8), anxiety (28.6%, n = 6), psychotic (23.8%, n = 5), and compulsive (4.8%, n = 1). Based on ICD-10 criteria,12 a comorbid psychiatric diagnosis could be made in 11 children (52.4%). Psychiatric diagnoses included organic mood disorder (F06.3) in 6, organic hallucinosis (F06.0) in 2, organic catatonic disorder (F06.1) in 2, and obsessive-compulsive disorder (F42.1) in 1. The proportion of postpubertal children with psychiatric diagnosis was more than that of prepubertal children (67% vs 42%).

- Psychiatric manifestations are common in pediatric N-methyl-d-aspartate receptor (NMDAR) encephalitis.
- Common psychiatric symptoms of pediatric NMDAR encephalitis include inappropriate crying behavior, social withdrawal, and unprovoked anger outburst.
- Prepubertal presentation of pediatric NMDAR encephalitis appears to be more severe and may lead to persistent psychiatric and cognitive symptoms.
- A high index of suspicion in children with sudden-onset behavioral issues with decreased use of speech, movement disorder, and seizure is crucial in early diagnosis and treatment of pediatric NMDAR encephalitis.
Speech disturbance in the form of decreased use of speech for communication was noted in all children except 3 (85.7%). Decreased use of limbs (47.6%, n = 10) and facial deviation (9.5%, n = 2) were also noted. A large proportion of the children had decreased sleep (71.4%, n = 15) and reduced food intake (61.9%, n = 13); 52.4% (n = 11) of the children also had double incontinence.
The majority of the children (85.7%, n = 18) had seizure (generalized tonic-clonic seizure: 71.4%, n = 15; focal seizure: 19%, n = 4; or myoclonic jerks: 4.8%, n = 1). Movement disorder was also seen in a majority of the children (76.2%, n = 16), the most common being facial involuntary movements in 10 (47.6%). Other movement disorders included choreiform movements (19%, n = 4), rigidity (14.3%, n = 3), dystonia (9.5%, n = 2), and unclassified (61.9%, n = 13). Electroencephalogram (EEG) abnormalities were found in 18 children, and magnetic resonance imaging brain abnormalities were found in 8. None of the patients were found to have a tumor.
Treatment Details
All 21 children received pulse methylprednisolone (30 mg/kg/d, maximum 1 g/d intravenous infusion over 4 to 5 hours for 5 days). Of these, 7 received only methylprednisolone as immune modulation. Thirteen children required additional immune modulation (due to lack of improvement or suboptimal improvement), 9 of those received plasmapheresis, and the other 4 children received intravenous immunoglobulin (1 received both). All children received antiepileptic drugs as follows: levetiracetam (n = 10), sodium valproate (n = 9), phenytoin (n = 6), clobazam (n = 6), carbamazepine (n = 4), oxcarbazepine (n = 2), and lacosamide (n = 1). These drugs were used only for the management of epilepsy and not for mood symptoms. Thirteen children (61.9%) required psychotropic medications for the management of behavioral symptoms. The most common psychotropic medications used were atypical antipsychotics (n = 10): quetiapine (n = 5), risperidone (n = 4), and olanzapine (n = 1), followed by benzodiazepines (n = 7): clonazepam (n = 5) and lorazepam (n = 2). One child each received haloperidol and fluoxetine. Eight children required no psychotropic medication.
Treatment Response
Follow-up details were available for 20 of the 21 children. Mean ± SD duration of follow-up was 20.8 ± 12.6 months and ranged from 4 to 50 months. Improvement in behavioral symptoms was found in all children during follow-up. In all children except 4, the BPRS-C score returned to zero (n = 16). Residual symptoms in those 4 children included irritability and anger outbursts (BPRS-C score = 3). The mean ± SD duration to achieve this significant clinical response was 7.4 ± 4.8 months and ranged from 2 to 19 months. In the 4 children with residual psychiatric symptoms, symptoms persisted until their last follow-up (mean ± SD = 17.3 ± 10 months; range, 6-28 months). Further, 3 of these 4 children were prepubertal. There was no relapse in the psychiatric symptoms during the entire follow-up period in any of the children. By first follow-up (mean ± SD = 4 ± 2.7 months; range, 1-12 months), seizure remission occurred in 15 (of 17, 88.2%), absence of involuntary movements in 11 (of 15, 73.3%), and improvement in use of speech in 13 (of 18, 72.2%). Four children (of 20, 20%) reported academic difficulties at first follow-up; 3 were prepubertal. These academic difficulties persisted for the entire duration of their follow-up (mean ± SD = 15.8 ± 7.1 months). Mean ± SD duration of continuation of immune modulation and antiepileptic medication in our sample was 21 ± 12 months. Mean ± SD duration of continuation of psychotropic medication was 13 ± 8 months. Major adverse effects noted during the course of treatment included diabetic ketoacidosis (n = 1), tubercular meningitis (n = 1), and weight gain (n = 4). No other major adverse events were reported.
DISCUSSION
In this study, we report that all children diagnosed with NMDAR encephalitis presented with psychiatric symptoms, the most common being inappropriate crying behavior, social withdrawal, and unprovoked anger outburst. Most of these common symptoms appear to be mood related. We also found that psychiatric disturbances were the first symptom of NMDAR encephalitis in almost half of the children. Psychiatric symptoms were found to respond to immune modulation. Further, we also report that a comorbid psychiatric diagnosis could be made in about half of the children.
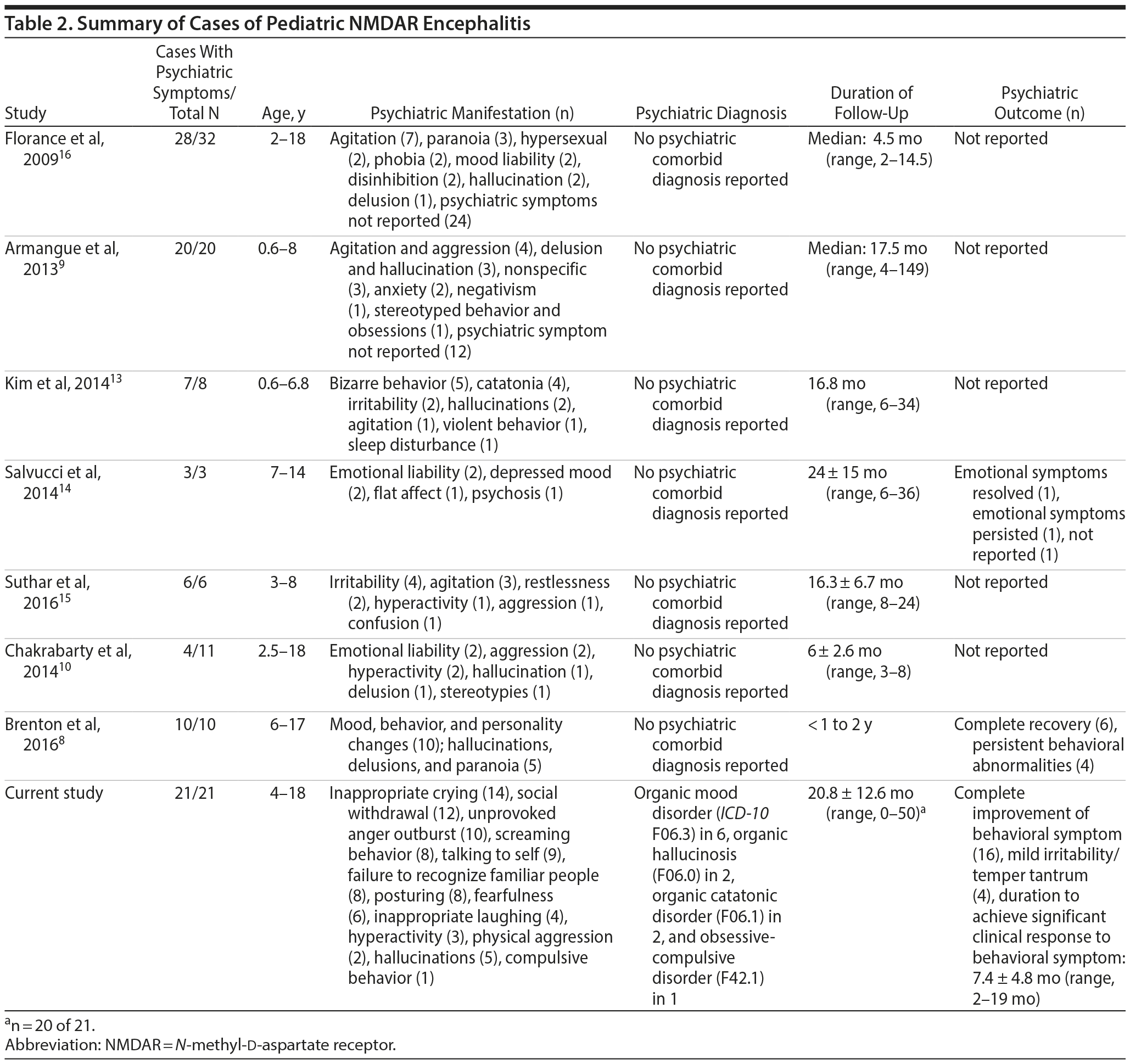
Our study concurs with the high frequency of psychiatric manifestations in pediatric NMDAR encephalitis reported in almost all of the previous case series8,9,13-16 (87.5%-100%) (Table 2). To our knowledge, only a single study10 has reported a lower frequency of psychiatric manifestations in this condition (36.4%). In the few studies10,14,15 with details of the psychiatric symptoms, irritability/emotional liability was commonly reported. These symptoms also appear to be mood related, which is similar to our study. Despite presence of mood symptoms, physical aggression was not common in our study. Associated reduced sleep and food intake were also noted to be frequent in our study.
Similar to our study, previous reports9,13,14,16 suggest that about half of the proportion of children with NMDAR encephalitis present with psychiatric manifestation as the first symptom. In addition to psychiatric symptoms described previously, all of the children in our study had at least 1 of the following at the initial presentation: seizure, movement disorder, or speech disturbance.
To our knowledge, no previous reports have systematically documented the presence of a comorbid psychiatric diagnosis in pediatric NMDAR encephalitis. Half of the children in our study had a comorbid psychiatric disorder, with organic mood disorder being the most common. Further, no previous studies have explicitly described the outcome of psychiatric manifestations when they reported treatment outcome. Improvement in psychiatric symptoms was noted in our study soon after initiation of immune modulation, with almost complete remission within a few months. This improvement was also maintained thereafter. This finding suggests that the psychiatric manifestations in this condition are responsive to immune modulation. All children were maintained on long-term immune modulation. In all children except 2 (tuberculous meningitis and diabetic ketoacidosis), no major adverse events were reported during the course of treatment. Although a significant proportion of children also required a short course of psychotropic medication (commonly atypical antipsychotics and benzodiazepines), 38.1% required no psychotropic medication for their behavioral symptoms.
Although we found no statistically significant differences between prepubertal and postpubertal children in our cohort, we did find some trends (see Table 1). Prepubertal children had a greater severity and longer duration of illness at presentation. In addition, the majority of the children with residual psychiatric symptoms and persistent academic difficulties were from the prepubertal group. In contrast, the finding that psychiatric symptom as the first manifestation was more common in the postpubertal children is similar to a previous report.9 This finding might partly explain why a comorbid psychiatric diagnosis was more commonly made in the postpubertal children.
Taken together with published literature,8-10,13-16 these findings underline the need to have a high index of suspicion in children with sudden-onset behavioral issues (especially mood-related symptoms). Given that early treatment has been found to be a predictor for better outcome in pediatric NMDAR encephalitis,17 the need to search for clues like decreased use of speech, movement disorder, and seizure in such cases is of utmost importance. This study has many limitations, including being medical record based and having a small sample size. Larger, systematic, prospective, longitudinal studies will help to elucidate the psychiatric aspects of pediatric NMDAR encephalitis.
Submitted: February 8, 2017; accepted May 5, 2017.
Published online: August 17, 2017.
Potential conflicts of interest: None.
Funding/support: None.
REFERENCES
1. Dalmau J, T×¼z×¼n E, Wu HY, et al. Paraneoplastic anti-N-methyl-d-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36. PubMed doi:10.1002/ana.21050
2. Gable MS, Sheriff H, Dalmau J, et al. The frequency of autoimmune N-methyl-d-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin Infect Dis. 2012;54(7):899-904. PubMed doi:10.1093/cid/cir1038
3. Lebon S, Mayor-Dubois C, Popea I, et al. Anti-N-methyl-d-aspartate (NMDA) receptor encephalitis mimicking a primary psychiatric disorder in an adolescent. J Child Neurol. 2012;27(12):1607-1610. PubMed doi:10.1177/0883073812438099
4. Rozier M, Morita D, King M. Anti-N-methyl-d-aspartate receptor encephalitis: a potential mimic of neuroleptic malignant syndrome. Pediatr Neurol. 2016;63:71-72. PubMed doi:10.1016/j.pediatrneurol.2016.03.023
5. Seifi A, Xia BT, Felte RF. Thinking outside the box about young female patients with sudden-onset bizarre behavior: a case of anti-N-methyl-d-aspartate receptor encephalitis. Prim Care Companion CNS Disord. 2013;15(4):PCC.13l01521.
6. Nagappa M, Bindu PS, Mahadevan A, et al. Clinical features, therapeutic response, and follow-up in pediatric anti-N-methyl-d-aspartate receptor encephalitis: experience from a tertiary care university hospital in India. Neuropediatrics. 2016;47(1):24-32. PubMed doi:10.1055/s-0035-1569464
7. Kamble N, Netravathi M, Saini J, et al. Clinical and imaging characteristics of 16 patients with autoimmune neuronal synaptic encephalitis. Neurol India. 2015;63(5):687-696. PubMed doi:10.4103/0028-3886.166532
8. Brenton JN, Kim J, Schwartz RH. Approach to the management of pediatric-onset anti-N-methyl-d-aspartate (Anti-NMDA) receptor encephalitis: a case series. J Child Neurol. 2016;31(9):1150-1155. PubMed doi:10.1177/0883073816643406
9. Armangue T, Titulaer MJ, Mסlaga I, et al; Spanish Anti-N-methyl-d-Aspartate Receptor (NMDAR) Encephalitis Work Group. Pediatric anti-N-methyl-d-aspartate receptor encephalitis-clinical analysis and novel findings in a series of 20 patients. J Pediatr. 2013;162(4):850-856.e2. PubMed doi:10.1016/j.jpeds.2012.10.011
10. Chakrabarty B, Tripathi M, Gulati S, et al. Pediatric anti-N-methyl-d-aspartate (NMDA) receptor encephalitis: experience of a tertiary care teaching center from north India. J Child Neurol. 2014;29(11):1453-1459. PubMed doi:10.1177/0883073813494474
11. Mullins D, Pfefferbaum B, Schultz H, et al. Brief Psychiatric Rating Scale for Children: quantitative scoring of medical records. Psychiatry Res. 1986;19(1):43-49. PubMed doi:10.1016/0165-1781(86)90091-0
12. World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva, Switzerland: World Health Organization; 1992.
13. Kim SY, Choi SA, Ryu HW, et al. Screening autoimmune anti-neuronal antibodies in pediatric patients with suspected autoimmune encephalitis. J Epilepsy Res. 2014;4(2):55-61. PubMed doi:10.14581/jer.14012
14. Salvucci A, Devine IM, Hammond D, et al. Pediatric anti-NMDA (N-methyl-d-aspartate) receptor encephalitis. Pediatr Neurol. 2014;50(5):507-510. PubMed doi:10.1016/j.pediatrneurol.2014.01.012
15. Suthar R, Saini AG, Sankhyan N, et al. Childhood anti-NMDA receptor encephalitis. Indian J Pediatr. 2016;83(7):628-633. PubMed doi:10.1007/s12098-015-1988-8
16. Florance NR, Davis RL, Lam C, et al. Anti-N-methyl-d-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009;66(1):11-18. PubMed doi:10.1002/ana.21756
17. Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157-165. PubMed doi:10.1016/S1474-4422(12)70310-1
Enjoy this premium PDF as part of your membership benefits!